Abstract
OBJECTIVES
To explore factors influencing functional status over time after cancer surgery in adults aged 65 and older.
DESIGN
Secondary data analysis of combined data subsets.
SETTING
Five prospective, longitudinal oncology nurse-directed clinical studies conducted at three academic centers in the northwest and northeast United States.
PARTICIPANTS
Three hundred sixteen community-residing patients diagnosed with digestive system, thoracic, genitourinary, and gynecological cancers treated primarily with surgery.
MEASUREMENTS
Functional status, defined as performance of current life roles, was measured using the Enforced Social Dependency Scale and the Medical Outcomes Study 36-item Short-Form Survey (using physical component summary measures) after surgery (baseline) and again at 3 and 6 months. Number of symptoms, measured using the Symptom Distress Scale, quantified the effect of each additional common cancer symptom on functional status.
RESULTS
After controlling for cancer site and stage, comorbidities, symptoms, psychological status, treatment, and demographic variables, functional status was found to be significantly better at 3 and 6 months after surgery than at baseline. Factors associated with better functional status included higher income and better mental health. Factors associated with poorer average functional status were a greater number of symptoms and comorbidities. Persons reporting three or more symptoms experienced statistically significant and clinically meaningful poorer functional status than those without symptoms. Persons reporting three or more comorbidities were also found to have poorer functional status than those without comorbidities. No significant relationship existed between age and functional status in patients aged 65 and older.
CONCLUSION
Factors other than age affect recovery of functional status in older adults after cancer surgery.
Keywords: functional status, older adult, cancer surgery
Adults aged 65 and older account for 60% of all newly diagnosed solid cancers and 80% of all cancer deaths.1 Functional status of older adults serves as an important healthcare indicator and research outcome. Loss of functional status is associated with shorter survival, compromised quality of life, depression, and severe economical burden for patients and their caregivers.2 Thus, a major healthcare goal for older adults with cancer consists of preserving, regaining, or attaining maximal functional status while undergoing cancer treatment.3
Surgery, a major component of cancer treatment in older adults, may affect functional status in some manner, but research has not clearly characterized older adults’ functional status after cancer surgery or the factors that influence it.4,5 This remains a critical gap in the literature; evidence regarding the functional status of older adults after cancer surgery can potentially inform practice, target clinical resources, and advance future research to improve care for older adults with cancer.
The purpose of this study was therefore to explore factors that influence functional status of older adults after surgery for thoracic, abdominal, or pelvic cancer using secondary data from five nurse-directed clinical oncology studies investigating the effect of care provided by advanced practice nurses (APNs) after surgery. The specific aims of the study were to describe the patient characteristics of the study sample, describe mean functional status at baseline and 3 and 6 months according to age group and cancer category, and explore factors that are significantly associated with functional status over time in a multivariable model with age and cancer as the main variables of interest. In conducting this study, functional status was broadly defined as the individual’s performance of activities associated with current life roles. This definition incorporates the roles of physical and social components in determining older adults’ functional status.3,6 Data from subsets of subjects aged 65 and older from the five nurse-directed clinical studies were combined to increase the population size to improve the power to detect differences.
METHODS
Design
A secondary data analysis was conducted using data from five nurse-directed randomized clinical trials investigating the effect of care provided by APNs after surgery. The Vulnerability/Risk/Human Response care model of nursing, an ecological model developed from the biopsychosocial point of view, guided the selection of independent and dependent variables for this study.7 The model describes the effect of the interaction between individual vulnerability (demographic, biological, psychological) and risk and support (treatment) factors on human responses (functional status).
The Yale School of Nursing Human Subjects Institutional Review Board approved the current study. Informed consent had been previously obtained from all patients during the parent studies, and study identification numbers were used in place of names or personal identifying data to protect the rights of human subjects.
Data Sources
The five clinical studies were conducted between 1983 and 2007 at academic cancer centers in the northwest and northeast United States. Complete details of these studies have been provided elsewhere.8–12 Patients were recruited during hospitalization and baseline interviews conducted within 1 month after discharge except for Study 1, in which patients were recruited in outpatient clinics after discharge, and baseline interviews were conducted on average 60 days after surgery. The data collection times consisted of baseline (enrollment) and 3 and 6 months after enrollment. Demographic data and comorbidities were recorded at baseline. Cancer site, stage, and treatment data were collected from treatment records and medical record audits. Functional status, symptoms, and mental health data were obtained from patient interviews at baseline and 3 and 6 months. The types of cancers differed between studies. Study 1 consisted of patients with thorax cancers. Study 2 and 3 enrolled patients with breast, colorectal, head and neck, lung, prostate, gynecological, bladder, pancreatic, esophageal, renal, and gastric cancers. Study 4 targeted men with prostate cancer. The Study 5 patient population consisted of women undergoing abdominal surgery for presumed gynecological cancers; ultimately, the final pathology revealed a heterogeneous group of cancers, including ovarian, uterine, endometrial, metastatic breast, and metastatic pancreatic cancers.
For the analysis, the cancer types were grouped based on the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, Sixth Edition, classification system13 and were categorized as breast, digestive system (esophageal, gastric, colorectal, pancreatic), thoracic (lung), gynecological (vulva, uterine, ovarian), and genitourinary (prostate, renal, pelvis, ureter, bladder). These cancer categories are documented to have greater incidence in older adults.14
Participant Selection
One thousand thirty-two participants were enrolled between the five studies, of whom 537 were aged 65 and older (Table 1). Because of subject attrition, 413 patients had data collected for a minimum of two or more time points. Because relationships between independent and dependent variables are best analyzed in homogenous populations, the study population was limited to 316 subjects aged 65 and older diagnosed with digestive system, thoracic, gynecological, and genitourinary treated primarily with surgery, enrolled in five nurse-directed randomized studies, with no other concurrent malignancy or other medical treatment and a minimum of two points of data collection. Surgical data were available for 189 patients; 186 of these were listed as undergoing surgical resection of their tumors, and three were treated with colonoscopy, biopsy, or lumpectomy. Because the population of interest for the original studies was patients undergoing cancer surgery, it was assumed that surgical data were missing or not collected for the remaining 127 patient. Hence, the final total sample for the current study consisted of 316 patients.
Table 1.
Participant Selection
| Participants | n (%) | |||||
|---|---|---|---|---|---|---|
| Study 18 | Study 29 | Study 310 | Study 411 | Study 512 | Total | |
| All | 165 (16.0) | 232 (22.5) | 383 (37.1) | 107 (10.4) | 145 (14.1) | 1,032 (100.0) |
| Aged ≥65 | 81 (15.1) | 116 (21.6) | 263 (49.0) | 23 (4.3) | 54 (10.1) | 537 (100.0) |
| With ≥2 observations | 42 (10.2) | 56 (13.6) | 243 (58.8) | 23 (5.6) | 49 (11.9) | 413 (100.0) |
| With ≥2 observations at four cancer sites | 42 (13.3) | 39 (12.3) | 169 (53.5) | 23 (7.3) | 43 (13.6) | 316 (100.0) |
Main Outcome Measure
The outcome variable for the study was functional status, which was conceptualized as representing the total domain of function (capacity, reserve, performance, and capacity utilization)6 and defined as the individuals’ performance of activities and tasks associated with their current life roles.3 Measures for functional status were the Enforced Social Dependency Scale (ESDS)15 and Medical Outcomes Study 36-item Short-Form Survey (SF-36)16–18 using the physical component summary measures.
The ESDS consists of personal and social competence. Personal competence includes six activities: eating, dressing, walking, traveling, bathing, and toileting. The patient reported dependency in each activity, which the interviewer rated on a 6-point scale. Scores for personal competence were summed and ranged from 6 to 36. Social competence consisted of home, work, and recreational activities, which were rated on 4-point scales, and communication, rated on a 3-point scale. Scores for social competence were summed and ranged from 4 to 15. Scores for personal and social competence were summed to generate a total dependency score ranging from 10 to 51, with higher scores reflecting greater dependency. The ESDS has demonstrated reliability (Cronbach α = 0.72–0.96) and validity.3,10,15
The SF-36 is a 36-item survey of health status used to assess eight health concepts: physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health. The SF-36 has demonstrated item internal consistency and item discriminant validity. Reliability coefficients have ranged from a low of 0.65 to a high of 0.94 across scales (median 0.85).17–19 The measure has also demonstrated validity in discriminating between patient groups with differing severity of medical and psychiatric illnesses.17
Although the five studies used similar longitudinal, repeated measures and study procedures, the functional status measures differed between studies; all studies used the ESDS except Study 4, which measured functional status with the SF-36. Because of the compatible study design, the studies were combined based on methods described in the literature. 20,21 These methods support that data from conceptually compatible studies with differing outcome measures can be combined using standardized scores. The data were therefore prepared for this study by reverse-coding the ESDS scores to attain consistent direction with the SF-36. The data were then converted to standardized norm-based scores (mean 50 ± 10) using 0 to 100 metric and z-scores.22 SF-36 scores were converted to norm-based scores using QualityMetric Health Outcomes Scoring Software 2.0 (QualityMetric Inc., Lincoln, RI).
Covariates
Demographic
The demographic variables for this study were age, sex, race or ethnicity, marital status, employment, religion, occupation, education, income, and living status. These were abstracted from the patient history forms and recorded on a standardized form. All subjects aged 65 and older during the 6 months of the study were included, and ages at 6 months were used for the analysis. Age categories typically used in gerontology research were initially used for the present study (65–74, 75–84, and ≥85). A review of the univariate statistics of the demographics revealed only 11 subjects (3.5% of population) aged 85 and older, so the categories were collapsed to 65 to 69, 70 to 74, and 75 and older to provide balance for the statistical analysis.
Biological Variables
The biological variables were number of symptoms, cancer stage, and comorbidities. Number of symptoms was measured according to the Symptom Distress Scale (SDS)23 to quantify the effect of each additional symptom on functional status. The SDS consists of 13 common symptoms of patients with cancer: frequency of nausea, severity of nausea, appetite, insomnia, frequency of pain, severity of pain, fatigue, bowel pattern, concentration, appearance, breathing, outlook, and cough. Subjects rated their distress on a scale from 1 (low distress) to 5 (high distress). Symptoms were considered present if patients’ rated their distress from 3 to 5 and absent if they rated them from 1 to 2. This categorization is consistent with previous studies using these same values to distinguish between low and high symptom distress.24 Number of symptoms, therefore, represented the sum of present symptoms, with the highest score equal to 13 and the lowest score equal to 0. The SDS has demonstrated validity and reliability, with reported Cronbach alphas ranging from 0.70 to 0.89.9,23,25
Cancer site and stage data were obtained from the original study medical record audits. Precedent from original studies was followed, and the same stage categories were used (early and late). For all cancers except prostate, early stage was defined as Stages I and II, and late stage was defined as Stages III and IV. In prostate cancer, early stage was defined as I, II, and III, and late stage was defined as IV. Because of advances in science, pathological reports varied in detail across the studies. For consistency, all reports were analyzed, compared, and updated to the current classification and staging system published in the AJCC Staging Manual, Sixth Edition,13 and the 2008 National Comprehensive Cancer Network Web site (http://www.nccn.org/clinical.asp). This review changed the stages of 11 study participants from early to late. A second independent coder reviewed and verified these changes. Chi-square analysis demonstrated no significant age differences between stage categories.
Comorbidity was defined as a preexisting health condition or disease other than the index cancer. Following procedures from the five original studies, all preexisting health conditions or diseases that the study participants reported were classified according to the following categories: cardiovascular; respiratory; endocrine; eye, ear, nose, or throat; psychiatric; neurological; genitourinary; gastrointestinal; skin; cancer; injuries; infectious; and other. The empirical indicator was sum of comorbidities. Evidence from research studies supports the concurrent and predictive validity of count of disease as a measure of comorbidity.26
Psychological
The health measures used in the five studies were the SF-36 Mental Component Summary scale, SF-12 Mental Component Summary scale, Profile of Mood States, Mental Health Inventory 5-item version, and Center for Epidemiologic Studies Depression Scale.16–18,22,27–29 Although multiple scales were used across studies, each had one consistent measurement item: “feeling blue or downhearted,” which was used for this study. This item is reported to be a powerful, nonspecific detector of mental health disorders.28
Treatments
Cancer treatment data were extracted from treatment records and medical record audits from the original studies. All patients in this study underwent surgery as their primary cancer treatment but may have also received chemotherapy, radiation therapy, or both. Hormone therapy was classified as chemotherapy in the original medical record audits, and this definition was maintained for the present study. Three patients started chemotherapy more than 3 months after enrollment. None were documented as receiving neo-adjuvant chemotherapy. Nursing intervention was defined as participation in an oncology advanced practice nurse intervention and operationalized as an assignment of study participants or control. The measurement items for the study were advanced practice nurse versus no advanced practice nurse; surgery alone; surgery and chemotherapy; surgery and radiation therapy; and surgery, chemotherapy, and radiation therapy.
Statistical Analysis
Analyses were performed using SAS version 9.2 (SAS Institute, Inc., Cary, NC). Bivariate analysis and analysis of variance (ANOVA) statistics tested the relationship at baseline between variables within the conceptual model and the outcome variable, functional status. Because functional status was a continuous variable, Pearson correlation statistical analyses were used to analyze the correlation between continuous independent variables and functional status score, and ANOVA was used to evaluate the relationship between categorical variables and functional status. The results from these analyses revealed no problems with multicollinearity between variables and confirmed the variables to be included in the model.
Descriptive statistics with frequencies and means were used to characterize the study participants and their mean functional status at baseline and 3 and 6 months according to age and cancer category. The analysis was then conducted exploring factors influencing functional status of study participants at baseline and 3 and 6 months. Longitudinal data with repeated observations over time on each of the study participants necessitates consideration of the correlation of repeated observations to obtain valid inferences about regression coefficients.30 For this reason, multiple linear regression models fit by generalized estimate equations (GEEs) were used for the functional status outcome analyses. The primary reason for using a GEE method instead of a random effects model was to develop missing data weights to account for the missing data due to loss to follow-up, as described below. Technically, the weights are the inverse of 1 minus the probability of observing the data. The weighted GEE method allowed less-stringent assumptions to be made concerning the missing data process than would be made using a complete case analysis of a random effects model.31
The main variables of interest for this analysis were age, cancer sites, and time. The potentially confounding demographic, biological, psychological, and treatment variables were included as covariates. Dummy indicators were used to study time and interactions between time and age categories to examine time trends in the effects of covariates. Choice of variables for the model was based on scientific reasoning to avoid bias of parameter estimates.32 An inverse probability-of-censoring weighted estimator was used because of the rate of attrition over the three waves of data. To implement this method, contributions to the standard GEE fitted model were weighted inversely according to the probability of staying in the study.33,34 Such inverse probability weighting accounts for data that are potentially missing at random, which is a less stringent assumption than the missing completely at random assumption of the unweighted GEE fit estimators. Robust standard errors were used because weights were used. Missing covariate data were not adjusted for, resulting in 51 patients excluded from the multivariable analysis. Inferences concerning comparisons were made using the coefficients from the estimated models. All tests of significance were made using two-sided hypothesis tests and a 5% Type 1 error rate. Previously described methods guided the analysis of the false discovery rate.35
In addition, a sensitivity analysis was conducted to investigate whether the results were similar after controlling for baseline function or modeling change in function over time. First, the association between functional status and demographic, biological, psychological, and treatment variables was examined using baseline functional status as a covariate. The relationship among functional status and demographic, biological, psychological, and treatment variables was then analyzed using rate of change in functional status from baseline to 3 months and from 3 to 6 months as the outcome variable.
RESULTS
Patient Characteristics
The total sample consisted of 316 patients. Subject attrition occurred over the course of the study, with substantial patient mortality during the 6 months of each study in participants aged 65 and older (n = 76). Chi-square (χ2) analysis indicated no significant difference between age categories (χ2 = 1.31, P=.52). Deaths were confirmed according to death certificates. Analysis of the characteristics of the study sample showed an age range from 65 to 93 (mean 71.8 ± 5.4) (Table 2). The sample was divided across age categories with 44.9% of the sample aged 65 to 69, 33.2% aged 70 to 74, and 21.8% aged 75 and older. In addition, the sample was balanced between men (49.7%) and women (48.7%) (does not add to 100% because of missing data). Most subjects were white (76.6%) and had completed eighth grade or greater (90.8%).
Table 2.
Demographic, Biological, Psychological, and Treatment Characteristics of Study Sample (N = 316)*
| Characteristic | Value |
|---|---|
| Age, mean ± SD (range) | 71.8 ± 5.4 (65–93) |
| Age, n (%) | |
| 65–69 | 142 (44.9) |
| 70–74 | 105 (33.2) |
| ≥75 | 69 (21.8) |
| Sex, n (%) | |
| Male | 157 (49.7) |
| Female | 154 (48.7) |
| Race, n (%) | |
| White | 242 (76.6) |
| Black or other | 55 (17.4) |
| Marital status, n (%) | |
| Married or living with partner | 195 (61.7) |
| Not married | 120 (38.0) |
| Employment status, n (%) | |
| Not working, retired, or unemployed | 260 (82.3) |
| Employed | 56 (17.7) |
| Education, n (%) | |
| Grade 1–8 | 29 (9.2) |
| Grade 9–12 | 145 (45.9) |
| ≥Grade 12 | 142 (44.9) |
| Religion, n (%) | |
| Protestant | 144 (45.6) |
| Catholic | 106 (33.5) |
| Jewish or other | 65 (20.6) |
| Annual income, $, n (%) | |
| 0–19,999 | 97 (30.7) |
| 20,000–39,999 | 65 (20.6) |
| ≥40,000 | 80 (25.3) |
| Unknown | 74 (23.4) |
| Living alone, n (%) | |
| Yes | 85 (26.9) |
| No | 231 (73.1) |
| Cancer site, n (%) | |
| Digestive | 70 (22.2) |
| Thoracic | 86 (27.2) |
| Gynecological | 73 (23.1) |
| Genitourinary | 87 (27.5) |
| Stage, n (%) | |
| Early | 171 (54.1) |
| Late | 108 (34.2) |
| Unknown | 37 (11.7) |
| Number of comorbidities, n (%) | |
| 0 | 22 (7.0) |
| 1 | 58 (18.4) |
| 2 | 58 (18.4) |
| ≥3 | 152 (48.1) |
| Recurrence, n (%) | |
| New diagnosis | 275 (87.0) |
| Recurrent disease | 36 (11.4) |
| Number of symptoms at baseline, n (%) | |
| 0 | 35 (11.1) |
| 1 | 42 (13.3) |
| 2 | 39 (12.4) |
| ≥3 | 195 (61.9) |
| Mental health at baseline, mean ± SD (range)† | 3.6 ± 1.2 (1–5) |
| Treatment, n (%) | |
| Surgery | 149 (47.2) |
| Surgery and chemotherapy | 101 (32.0) |
| Surgery, chemotherapy, and radiation | 39 (12.3) |
| Surgery and radiation | 27 (8.5) |
| Advance practice nurse intervention, n (%) | |
| Yes | 171 (54.1) |
| No | 145 (45.9) |
Percentages may not total to 100 because of missing values and rounding.
SD = standard deviation.
5 represents better mental health.
The sample was also balanced across annual income categories, with 30.7% of the subjects earning $0 to $19,999, 20.6% earning $20,000 to $39,999, and 25.3% earning $40,000 and more. Income was unknown for 23.4% of the sample. Although income was balanced across all categories, only 17.7% were working part time or full time. Most participants (61.7%) reported being married or living with a partner, and 26.9% lived alone. Most subjects had newly diagnosed cancer (87.0%), and the sample was balanced across digestive system (22.2%), thoracic (27.2%), gynecological (23.1%), and genitourinary (27.5%). There was a high prevalence of comorbidities across the study sample, with 48.1% reporting three or more. Treatment for cancer included surgery, chemotherapy, radiation therapy, and hormone therapy, and 52.8% of subjects received a combination of therapies; 54.1% received care provided by APNs after surgery.
Mean Functional Status
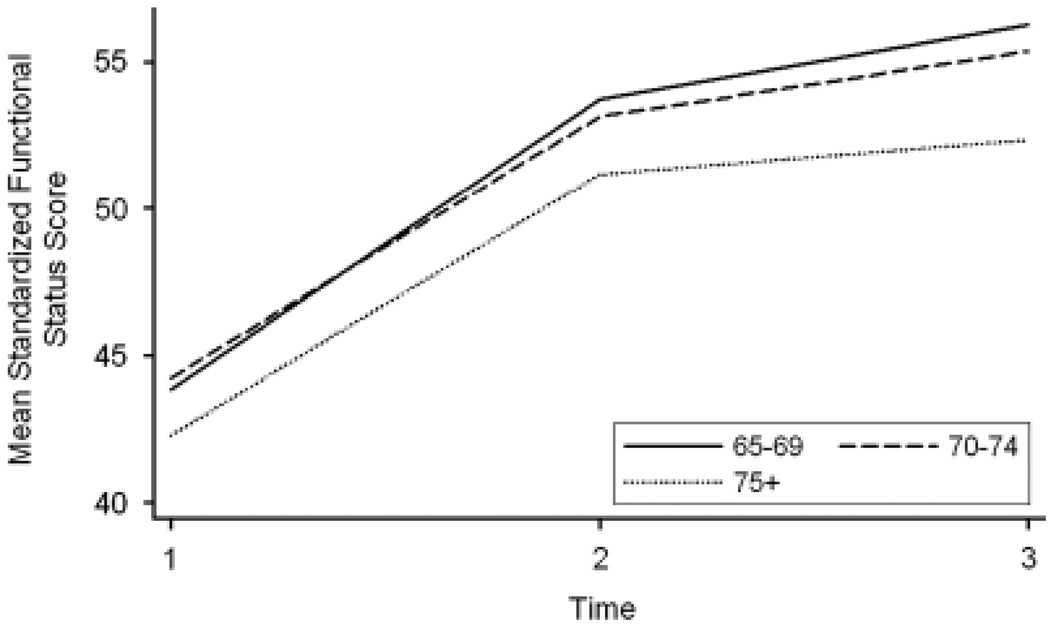
The mean functional status score over time was calculated for the study sample (Table 3 and Figure 1). These results demonstrated that, on average, the sample had better functional status at 3 and 6 months than at baseline. These improvements were expected because baseline measures were generally within 1 to 2 months after surgery. The mean functional status score was 43.6 ± 9.0 at baseline (n = 315, range 24.5–67.5), 53.0 ± 8.8 at 3 months (n = 306, range 25.7–67.5), and 55.2 ± 8.5 at 6 months (n = 269, range 23.6–67.5).
Table 3.
Total Function Scores According to Cancer Site and Age (N = 316)
| Cancer Site and Age | n; Mean ± Standard Deviation | ||
|---|---|---|---|
| Baseline | 3 Months | 6 Months | |
| Digestive | |||
| 65–69 | 21; 43.7 ± 10.0 | 20; 53.6 ± 10.3 | 15; 58.2 ± 5.6 |
| 70–74 | 27; 43.0 ± 9.0 | 27; 53.8 ± 7.1 | 25; 55.3 ± 8.1 |
| ≥75 | 22; 40.8 ± 7.8 | 22; 48.3 ± 11.6 | 15; 55.4 ± 7.5 |
| Thoracic | |||
| 65–69 | 44; 45.7 ± 9.1 | 40; 52.6 ± 9.1 | 37; 53.6 ± 9.8 |
| 70–74 | 25; 47.9 ± 8.2 | 24; 53.3 ± 7.7 | 23; 53.1 ± 10.0 |
| ≥75 | 17; 48.2 ± 12.7 | 17; 50.3 ± 11.8 | 13; 45.9 ± 12.2 |
| Gynecological | |||
| 65–69 | 29; 41.7 ± 7.4 | 29; 52.8 ± 7.7 | 21; 56.3 ± 8.2 |
| 70–74 | 22; 42.1 ± 7.7 | 22; 52.6 ± 7.5 | 21; 55.6 ± 6.9 |
| ≥75 | 22; 38.8 ± 7.6 | 22; 53.5 ± 9.5 | 20; 53.7 ± 8.3 |
| Genitourinary | |||
| 65–69 | 48; 43.5 ± 7.6 | 45; 55.3 ± 7.7 | 45; 57.8 ± 6.6 |
| 70–74 | 30; 43.9 ± 10.2 | 30; 52.8 ± 8.1 | 28; 57.2 ± 6.8 |
| ≥75 | 8; 43.4 ± 8.2 | 8; 54.3 ± 7.7 | 6; 54.1 ± 9.4 |
| Total population | 315; 43.6 ± 9.0 | 306; 53.0 ± 8.8 | 269; 55.2 ± 8.5 |
Higher scores indicate better functional status.
Total may not equal to 316 because of missing data.
Figure 1.
Mean Medical Outcomes Study 36-item Short-Form Survey physical component summary score 50 ± 10 (possible range 2–76, but extreme scores are unlikely).50 Mean Enforced Social Dependency Scale score 50 ± 10 (possible range 20.2–67.5). Functional status score range 23.6 to 67.5. Time 1 = baseline data collection, Time 2 = data collection at 3 months, Time 3 = data collection at 6 months.
At baseline, patients with gynecological cancer aged 75 and older had the lowest mean functional status score (38.8 ± 7.6, n = 22), and those aged 75 and older with thoracic cancer reported the highest mean functional status score (48.2 ± 12.7, n = 17). At 3 months, patients aged 65 to 69 with genitourinary cancer reported the highest mean functional status score (55.3 ± 7.7, n = 45), and patients aged 75 and older with digestive cancer had the lowest mean functional status score (48.3 ± 11.6, n = 22). Patients aged 65 to 69 with digestive cancer had the highest mean functional status score (58.2 ± 5.6, n = 15), and those aged 75 and over with thoracic cancer had the lowest mean functional status score (45.9 ± 12.2, n = 13) at 6 months.
Factors Associated with Functional Status
Factors associated with functional status in older adults after surgery for digestive system, thoracic, gynecological, and genitourinary cancer were then explored while controlling for demographic, biological, psychological, and treatment variables at baseline and longitudinally at 3 and 6 months (Table 4). Results from the analysis revealed that chronological age was not significantly associated with functional status over time, although there were statistically significant relationships between functional status, cancer category, time, income, treatment, comorbidities, total number of symptoms, and mental health.
Table 4.
Relationship Between Functional Status and Age, Controlling for Demographic, Biological, Psychological, and Treatment Variables (N = 265)
| Characteristic | Parameter (Standard Error) | 95% Confidence Interval | P-Value |
|---|---|---|---|
| Age | |||
| 65–69 | Reference | — | — |
| 70–74 | −0.24 (1.03) | −2.25–1.78 | .82 |
| ≥75 | −1.40 (1.25) | −3.85–1.06 | .26 |
| Cancer site | |||
| Digestive | 0.49 (0.87) | −1.22–2.20 | .57 |
| Thoracic | 1.97 (0.81) | 0.38–3.56 | .01 |
| Gynecological | 1.24 (1.05) | −0.82–3.30 | .24 |
| Genitourinary | Reference | — | — |
| Time | |||
| Baseline | Reference | — | — |
| 3 months | 8.97 (0.93) | 7.14–10.81 | <.001 |
| 6 months | 10.12 (1.01) | 8.15–12.09 | <.001 |
| Annual income, $ | |||
| 0–19,999 | Reference | — | — |
| 20,000–39,999 | 0.92 (0.88) | −0.82–2.65 | .30 |
| ≥40,000 | 2.82 (0.90) | 1.06–4.57 | .002 |
| Unknown | 0.94 (0.90) | −0.83–2.71 | .30 |
| Number of comorbidities | |||
| 0 | Reference | — | — |
| 1 | −1.12 (1.39) | −3.85–1.62 | .42 |
| 2 | −1.35 (1.31) | −3.91–1.20 | .30 |
| ≥3 | −3.78 (1.24) | −6.20 to −1.36 | .002 |
| Number of symptoms | |||
| 0 | Reference | — | — |
| 1 | −2.06 (0.59) | −3.21 to −0.91 | <.001 |
| 2 | −3.95 (0.85) | −5.62 to −2.28 | <.001 |
| ≥3 | −6.05 (0.69) | −7.39 to −4.71 | <.001 |
| Mental health | 0.84 (0.26) | 0.34–1.34 | .001 |
| Treatment | |||
| Surgery | Reference | — | — |
| Surgery and chemotherapy | −1.61 (0.73) | −3.04 to −0.18 | .03 |
| Surgery, chemotherapy, and radiation | −0.04 (0.88) | −1.76–1.68 | .96 |
| Surgery and radiation | 0.01 (1.29) | −2.51–2.54 | .99 |
Significant parameters only except age variables.
Total not equal to 316 because of missing data.
Time of data collection, cancer category, income, and mental health were significantly related to better average functional status. For time of data collection, the results demonstrated that functional status of subjects was better at 3 months (β = 8.97, 95% confidence interval (CI) = 7.14–10.81, P<.001) and 6 months (β = 10.12, 95% CI = 8.15–12.09, P<.001) than at baseline. Patients with thoracic cancer reported better functional status than those with genitourinary cancers (β = 1.97, 95% CI = 0.38–3.56, P = .01). Subjects who earned $40,000 and more annually had better functional status than those earning less than $20,000 (β = 2.82, 95% CI = 1.06–4.57, P=.002). Those who reported better mental health also reported better functional status (β = 0.84, 95% CI = 0.34–1.34, P=.001).
In contrast, the covariates treatment, comorbidities, and symptoms were significantly related to poorer average functional status. Patients who received a combination of surgery and chemotherapy reported poorer functional status than those who underwent surgery alone (β = −1.61, 95% CI = −3.04 to −0.18, P=.03). For comorbidities, patients who had three or more comorbidities had poorer functional status than those who reported no comorbidities (β = −3.78, 95% CI = −6.20 to −1.36, P=.002). Patients who reported one (β = −2.06, 95% CI = −3.21 to −0.91, P<.001), two (β = −3.95, 95% CI = −5.62 to −2.28, P<.001), and three or more symptoms (β = −6.05, 95% CI = −7.39 to −4.71, P<.001) also reported poorer functional status than those without symptoms.
All of the results were statistically significant if a 10% false discovery rate was assumed. With 316 people and standardized outcome and covariates (all standardized to have the same variance), there would be 80% power to detect a correlation or slope between a covariate and the outcome of 0.16 using a simple linear regression. This assumes the use of two-sided hypothesis tests and 5% Type 1 error rates. A correlation of 0.16 is often considered relatively modest.36
Sensitivity Analysis
Whether the inferences concerning functional status and demographic, biological, psychological, and treatment variables changed if baseline functional status was included as a covariate in the model was first investigated. Results from this analysis again revealed that chronological age was not significantly associated with functional status over time, although there were statistically significant relationships between functional status and time of data collection, mental health, number of symptoms, and cancer category and stage. Patients reported significantly better functional status at 6 months than at 3 months (β = 1.19, 95% CI = 0.09– 2.38, P=.048). Patients with digestive cancer reported better functional status than those with genitourinary cancer (β = 2.18, 95% CI = 0.07–4.28, P=.04). In addition, patients reporting better mental health reported better functional status (β = 0.72, 95% CI = 0.08–1.37, P=.03). In contrast, patients who reported one (β = −2.58, 95% CI = −3.84 to −1.31, P<.001), two (β = −4.60, 95% CI = −6.43 to −2.78, P<.001), and three or more (β = −5.64, 95% CI = −7.26 to −4.02, P<.001) symptoms also reported poorer functional status than those reporting no symptoms. Patients with late-stage cancers also reported poorer functional status than those with unknown- or early-stage cancers (β = −2.06, 95% CI = −3.72 to −0.41, P=.01).
Next, whether the inferences were different when examining change in functional status from baseline to 3 months and from 3 to 6 months was investigated. Results from this analysis again demonstrated that chronological age was not significantly associated with rate of change in functional status. One factor, unknown income, was positively associated with better rate of change in functional status. Time of data collection, symptoms, and cancer stage were associated with lower rate of change in functional status. Participants reporting unknown income experienced higher rate of change in functional status than those reporting income less than $20,000 (β = 2.13, 95% CI = 0.30–4.0, P=.02). In contrast, the rate of change in functional status was less between 3 and 6 months than between baseline and 3 months (β = −11.19, 95% CI = −13.57 to −8.81, P<.001). Participants who reported one (β = −2.06, 95% CI = −4.13–0.00, P=.050) and three or more symptoms (β = −4.44, 95%CI = −6.23 to −2.66, P<.001) also experienced lower rate of change than those without symptoms. Additionally, study participants with late-stage cancers reported lower rate of change in functional status over time than those with unknown- or early-stage cancers (β = −2.02, 95% CI = −3.36 to −0.68, P=.003).
DISCUSSION
Evidence suggests that some older adults with cancer may be denied standard surgical treatment out of concern for morbidity and mortality.37 To help clarify older adults’ tolerance of surgical procedures, the current study sought to identify factors affecting functional status of older adults after cancer surgery using secondary data from nurse-directed cancer clinical studies. Using time of data collection as a covariate provided information that, on average, older adults experienced a 10-point increase in functional status scores from baseline to 6 months after surgery. A previous study found that a 5-point difference in SF-36 scores indicated clinically significant change.38 This can also be used as a reference point for clinically significant change in the current study because, as with the SF-36, functional status scores were transformed to standardized norm-based scores. These results, therefore, suggest that older adults experienced a clinically meaningful recovery of functional status after cancer surgery, following a typical postsurgical recovery pattern.
Nevertheless, other factors were significantly associated with functional status after cancer surgery. The presence of three or more symptoms was associated with a 6-point lower functional status score. Again, using a 5-point difference between groups as a reference point, this represents a clinically significant difference in functional status scores. Comorbidities and combination treatment of surgery plus chemotherapy also decreased older adults’ average ability to recover functional status. Better mental health and income of $40,000 and more was associated with better functional status. Previous research4,5,39,40 supports these results showing an association between symptoms, comorbidities, treatment, mental health, income, and functional status.
One unexpected result from the analysis was that patients with thoracic cancers reported better functional status than study participants with genitourinary cancers. This is contrary to previous research in which patients with cancers of the thorax experienced worse functional status than those with prostate cancer.4 There may be several explanations for these results. In the current study, the majority of patients with thoracic cancer were primarily enrolled in Study 1, in which baseline data collection extended to 60 days after surgery. Thus, the functional status of these patients may have improved by the time of study enrollment. In addition, Study 1 was conducted before the adoption of combination treatments of surgery, chemotherapy, and radiation therapy for older patients with lung cancer.41 The patients with thoracic cancers, therefore, may have received less-aggressive treatment than other patients in the study. For example, 23.9% of all patients with lung cancer received chemotherapy, compared with 67% of gynecological patients, who were primarily enrolled in Study 5.
The current study results differ from research studies conducted in the general geriatric population, which demonstrate that hospitalized community-dwelling older adults are at risk for decline in functional status, poor long-term functional outcomes, and high 1-year mortality. Additionally, older age may be a predictor of decline in functional status during or after hospitalization,42–45 although some evidence suggests that older general surgical patients may follow a different functional status recovery pattern than those hospitalized for acute medical illness. A longitudinal study of patients aged 60 and older undergoing abdominal surgery showed that, in general, the functional status of study participants improved within 6 months after surgery and that chronological age was not related to functional recovery.46 The current study results demonstrated a non-significant trend toward poorer functional status in patients aged 75 and older than in those aged 65 to 69. Presurgical data were not available to analyze whether providers selected only the fittest older adults for aggressive cancer therapy, creating a nonrepresentative sample and washing away the age effect. Nevertheless, the modest size of the age category point estimates in the full model suggests that the trend toward poorer functional status, if proven significant, would have translated into minimal clinical difference in functional status between age groups.
Clinical Implications
This study provides important evidence to help guide clinical decisions in caring for older adults after surgery, an important component of cancer treatment. The results demonstrate that, on average, older adults experience clinically significant recovery after cancer surgery. Considering other research showing similar findings, evidence is mounting that age should not be a criterion in determining who can or cannot tolerate surgery.5,46,47 Additionally, the findings of the current study suggest that patients with three or more symptoms may be at greater risk for clinically significant poorer functional status than those without symptoms. With the association between functional status and morbidity after cancer surgery, patients with three or greater symptoms may be at greater risk for adverse outcomes.
These findings provide evidence to support perioperative symptom management that continues through surgical recovery and postoperative cancer treatments. This may include comprehensive assessment before surgery, ongoing management after surgery of common symptoms such as fatigue and pain, and close follow-up with primary provider and nurse home visits after discharge.4,10,48 The results of the current study support that older adults have the potential for a meaningful recovery with resources available to support effective symptom management strategies after cancer surgery.
Strengths and Limitations of Study
The strength of this study stems from the large size of the population as a result of using five combined studies, increasing the power of the study to detect clinically significant relationships. In addition, it avoided the challenge of recruiting older adults to clinical trials. A limitation is that even with the large size of the population, only 11 patients were aged 85 and older. Thus, this age group remains understudied.
Although the large size of the population strengthens the study, combining studies also created challenges. The 25-year span of the five combined studies may have confounded results because of historical events. Changes introduced into the clinical setting during the time span have included new cancer staging systems and treatments leading to better survival rates with less toxicity. Evolution in pathology and hospital data reporting and storage may have led to inconsistent data abstraction during medical record audits over time. Thus, recording of cancer stages and surgical procedures differed across studies. The initial analysis plan included use of dummy variables to represent each study as a control for the historical effect. These variables were ultimately removed from the model because of multicollinearity with the cancer site variables. In addition, because surgical cancer patients were the population of interest in the original studies, it was decided to include all patients in the analysis despite limited documentation of surgeries. To test whether this decision created any bias, the analysis was conducted with the full sample and with those with documented surgeries. The relationships were consistent across all variables, although there was an expected loss of statistical significance in the smaller sample for the covariates of treatment, income, and comorbidities. Changing subjects’ cancer stages to reflect the current AJCC staging system in addition to using cancer treatments as a covariate helped control for the historical effect.
This study also had limitations due to the use of secondary data. In the five original clinical studies, self-report measures were used to assess functional status. Evidence supports that performance-based measures may capture different functional limitations than self-report measures.46,49 Hence, use of performance-based and self-report measures may better capture the different dimensions of older adults’ functional status after cancer surgery, and future studies should include both types of measures.
Another limitation due to secondary analysis stemmed from differing functional status measures across studies. Methods for combining data sets are described in the literature and support that, after correcting for differing direction of scales through reverse coding, selected outcomes with standardized data from different studies can be used for comparison purposes when all items are related to a broadly defined concept such as functional status. Therefore, the data were combined to provide a more-robust sample and, consequently, the loss of potentially valuable information was prevented.20,21
CONCLUSION
The evidence supports that age does not significantly affect older adults’ ability to recover from surgery for thoracic, abdominal, or pelvic cancer. On average, older adults experience clinically significant improvement in functional status over time, although those who report three or more symptoms are at risk for significant worsening of functional status. Therefore, resources directed toward developing, testing, and implementing symptom management strategies in the clinical setting may improve older adults’ outcomes after cancer surgery.
ACKNOWLEDGMENTS
The authors would like to acknowledge the Yale Program on Aging for its contribution to the design and methods of the study.
Janet H. Van Cleave was funded in part by NewCourtland Center for Transitions and Health, University of Pennsylvania School of Nursing Postdoctoral Fellowship T32NR009356; John A. Hartford Foundation: Building Academic Geriatric Nursing Capacity Award Program; and Yale School of Nursing Predoctoral Fellowship T32NR008346. Brian Egleston was funded in part by National Institutes of Health Grant P30 CA006927 and an appropriation from the Commonwealth of Pennsylvania.
Sponsor’s Role: None.
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Author Contributions: Janet H. Van Cleave, Brian Egleston, and Ruth McCorkle: study concept, design, analysis, and interpretation of the data. Janet H. Van Cleave had full access to all the data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.NCCN Clinical Practice Guidelines in Oncology: Senior Adult Oncology v.1.2010. [Accessed July 1, 2010];National Comprehensive Cancer Network. doi: 10.6004/jnccn.2010.0007. [on-line], Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [DOI] [PubMed]
- 2.Luciani A, Jacobsen PB, Extermann M, et al. Fatigue and functional dependence in older cancer patients. Am J Clin Oncol. 2008;31:424–430. doi: 10.1097/COC.0b013e31816d915f. [DOI] [PubMed] [Google Scholar]
- 3.Richmond T, Tang ST, Tulman L, et al. Measuring function. In: Frank-Stromborg M, Olsen SJ, editors. Instruments for Clinical Health-Care Research. 3rd Ed. Sudbury, MA: Jones and Bartlett; 2004. pp. 83–99. [Google Scholar]
- 4.Given CW, Given B, Azzouz F, et al. Comparison of changes in physical functioning of elderly patients with new diagnoses of cancer. Med Care. 2000;38:482–493. doi: 10.1097/00005650-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson NA, Given CW. Determinants of functional recovery in older adults surgically treated for cancer. Cancer Nurs. 2004;27:10–16. doi: 10.1097/00002820-200401000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Leidy NK. Functional status and the forward progress of merry-go-rounds: Toward a coherent analytical framework. Nurs Res. 1994;43:196–202. [PubMed] [Google Scholar]
- 7.Shaver JF. A biopsychosocial view of human health. Nurs Outlook. 1985;33:186–191. [PubMed] [Google Scholar]
- 8.McCorkle R, Benoliel JQ, Donaldson G, et al. A randomized clinical trial of home nursing care for lung cancer patients. Cancer. 1989;64:1375–1382. doi: 10.1002/1097-0142(19890915)64:6<1375::aid-cncr2820640634>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.McCorkle R, Jepson C, Malone D, et al. The impact of posthospital home care on patients with cancer. Res Nurs Health. 1994;17:243–251. doi: 10.1002/nur.4770170403. [DOI] [PubMed] [Google Scholar]
- 10.McCorkle R, Strumpf NE, Nuamah IF, et al. A specialized home care intervention improves survival among older post-surgical cancer patients. J Am Geriatr Soc. 2000;48:1707–1713. doi: 10.1111/j.1532-5415.2000.tb03886.x. [DOI] [PubMed] [Google Scholar]
- 11.McCorkle R, Siefert ML, Dowd MF, et al. Effects of advanced practice nursing on patient and spouse depressive symptoms, sexual function, and marital interaction after radical prostatectomy. Urol Nurs. 2007;27:65–77. [PubMed] [Google Scholar]
- 12.McCorkle R, Dowd M, Ercolano E, et al. Effects of a nursing intervention on quality of life outcomes in post-surgical women with gynecological cancers. Psychooncology. 2009;18:62–70. doi: 10.1002/pon.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th Ed. New York: Springer; 2002. [Google Scholar]
- 14.Cancer Facts & Figures 2010. [Accessed July 2, 2010];American Cancer Society. [on-line], Available at http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/can cer-facts-and-figures-2010.
- 15.Tang S, McCorkle R. A User’s Manual for the Enforced Social Dependency Scale. New Haven, CT: Yale School of Nursing; 2002. [Google Scholar]
- 16.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 17.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 18.McHorney CA, Ware JE, Jr, Lu JF, et al. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kazis LE, Lee A, Spiro A, III, et al. Measurement comparisons of the medical outcomes study and veterans SF-36 health survey. Health Care Financ Rev. 2004;25:43–58. [PMC free article] [PubMed] [Google Scholar]
- 20.Orsi AJ, Grey M, Mahon MM, et al. Conceptual and technical considerations when combining large data sets. West J Nurs Res. 1999;21:130–142. doi: 10.1177/01939459922043785. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration; 2009. [Accessed July 3, 2010]. [updated September 2009], [on-line], Available at http://www.cochrane-handbook.org. [Google Scholar]
- 22.Ware JE, Jr, Kosinski M, Turner-Bowker DM, et al. How to Score Version 2 of the SF-12 Health Survey (With a Supplement Documenting Version 1) In: Lincoln RI, Boston MA, editors. QualityMetric Incorporated and Health Assessment Lab. 2005. [Google Scholar]
- 23.McCorkle R, Cooley ME, Shea J. A User’s Manual for the Symptom Distress Scale. New Haven, CT: Yale School of Nursing; 1998. [Google Scholar]
- 24.Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12:694–708. doi: 10.1002/pon.694. [DOI] [PubMed] [Google Scholar]
- 25.Sarna L, Lindsey AM, Dean H, et al. Weight change and lung cancer: Relationships with symptom distress, functional status, and smoking. Res Nurs Health. 1994;17:371–379. doi: 10.1002/nur.4770170508. [DOI] [PubMed] [Google Scholar]
- 26.de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity: A critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 27.McNair D, Lorr M, Droppleman L. Education and Industrial Testing Service Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing; 1971. [Google Scholar]
- 28.Berwick DM, Murphy JM, Goldman PA, et al. Performance of a five-item mental health screening test. Med Care. 1991;29:169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–1839. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
- 31.Hogan JW, Roy J, Korkontzelou C. Handling drop-out in longitudinal studies. Stat Med. 2004;23:1455–1497. doi: 10.1002/sim.1728. [DOI] [PubMed] [Google Scholar]
- 32.Whittingham MJ, Stephens PA, Bradbury RB, et al. Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol. 2006;75:1182–1189. doi: 10.1111/j.1365-2656.2006.01141.x. [DOI] [PubMed] [Google Scholar]
- 33.Fitzmaurice GM, Laird NM. Generalized linear mixture models for handling nonignorable dropouts in longitudinal studies. Biostatistics. 2000;1:141–156. doi: 10.1093/biostatistics/1.2.141. [DOI] [PubMed] [Google Scholar]
- 34.Scharfstein DO, Rotnitzky A, Robins JM. Adjusting for nonignorable dropout using semiparametric nonresponse models. J Am Stat Assoc. 1999;94:1096–1120. [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 36.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 37.Audisio RA, Bozzetti F, Gennari R, et al. The surgical management of elderly cancer patients: Recommendations of the SIOG surgical task force. Eur J Cancer. 2004;40:926–938. doi: 10.1016/j.ejca.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Kahn SR, Ducruet T, Lamping DL, et al. Prospective evaluation of health-related quality of life in patients with deep venous thrombosis. Arch Intern Med. 2005;165:1173–1178. doi: 10.1001/archinte.165.10.1173. [DOI] [PubMed] [Google Scholar]
- 39.Given B, Given C, Azzouz F, et al. Physical functioning of elderly cancer patients prior to diagnosis and following initial treatment. Nurs Res. 2001;50:222–232. doi: 10.1097/00006199-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Li AK, Covinsky KE, Sands LP, et al. Reports of financial disability predict functional decline and death in older patients discharged from the hospital. J Gen Intern Med. 2005;20:168–174. doi: 10.1111/j.1525-1497.2005.30315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunn PA, Jr, Lilenbaum R. Chemotherapy for elderly patients with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2003;95:341–343. doi: 10.1093/jnci/95.5.341. [DOI] [PubMed] [Google Scholar]
- 42.Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Arch Intern Med. 1996;156:645–652. [PubMed] [Google Scholar]
- 43.Gill TM, Allore HG, Holford TR, et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 44.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 45.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg. 2004;199:762–772. doi: 10.1016/j.jamcollsurg.2004.05.280. [DOI] [PubMed] [Google Scholar]
- 47.Mastracci TM, Hendren S, O’Connor B, et al. The impact of surgery for colorectal cancer on quality of life and functional status in the elderly. Dis Colon Rectum. 2006;49:1878–1884. doi: 10.1007/s10350-006-0725-9. [DOI] [PubMed] [Google Scholar]
- 48.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 49.Whitson HE, Sanders LL, Pieper CF, et al. Correlation between symptoms and function in older adults with comorbidity. J Am Geriatr Soc. 2009;57:676–682. doi: 10.1111/j.1532-5415.2009.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10:395–404. doi: 10.1023/a:1012552211996. [DOI] [PubMed] [Google Scholar]