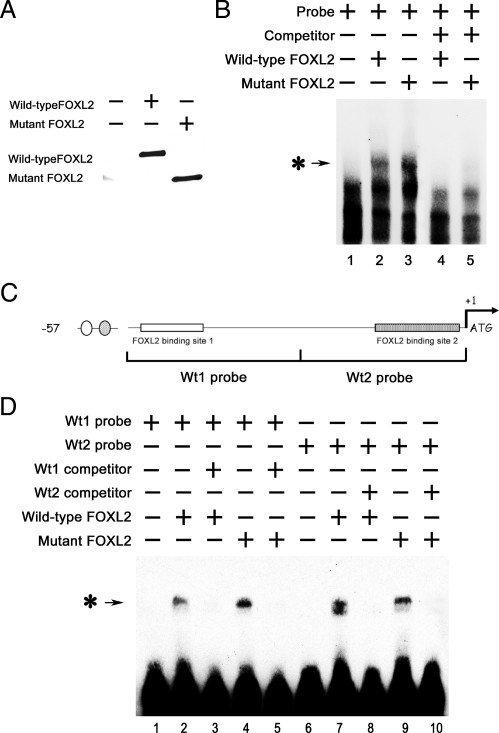
Fig. 4.
Wild-type and mutant FOXL2 bind to the −57-bp region of the CYP19 promoter. CHO cells were transfected with FLAG-tagged wild-type FOXL2 or mutant FOXL2, and after 24 h of transfection, FLAG-tagged proteins were immunoprecipitated. A, Western blot showing the relative amounts of wild-type and mutant FOXL2 protein expressed after transient transfection. B, An oligonucleotide probe corresponding to the −57-bp region of the CYP19 promoter was labeled with biotin and mixed with 3 μl of IP products from CHO cells that had been transfected with empty vector backbone (lane 1), wild-type FOXL2 (lane 2), mutant FOXL2 (lane 3), wild-type FOXL2 and an excess of competitor (non-biotin-labeled probe) (lane 4), or mutant FOXL2 and an excess of competitor (lane 5). These mixtures were then analyzed using the LightShift EMSA system. A protein-DNA complex band was detected when nuclear extracts from CHO cells expressing wild-type or mutant FOXL2 (asterisk-arrow) was combined with the −57-bp CYP19 promoter and was competed out by an excess of unlabeled probe. C, Schematic of the −57-bp CYP19 promoter indicating the two potential FOXL2-binding sites. Corresponding 30-bp biotin-labeled probes (wt1 and wt2 probes) and non-biotin-labeled competitors (wt1 and wt2 competitors) were synthesized. D, Wt1 and wt2 probes (lanes 1–5 and 6–10, respectively) were mixed with wild-type (lanes 2 and 7) or mutant (lanes 4 and 9) FOXL2 or with wild-type (lanes 3 and 8) or mutant FOXL2 (lanes 5 and 10) and excess amounts of competitors. A protein-DNA complex band was detected in mixtures containing wild-type and/or mutant FOXL2 and either the wt1 or wt2 probe (asterisk-arrow), suggesting that both wild-type and mutant FOXL2 can bind to both binding sites.