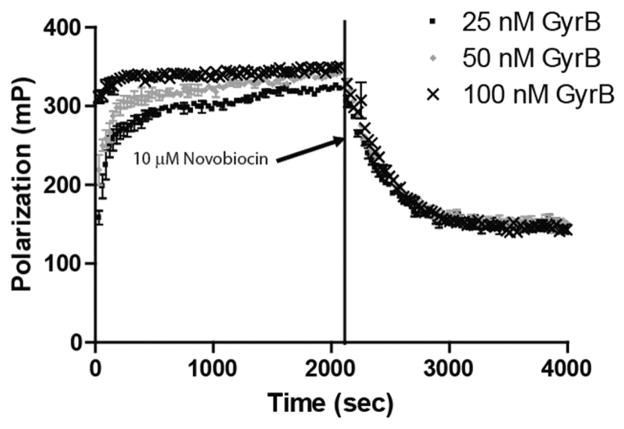
FIG. 1.
Equilibration studies of the interaction of Novo-TRX and GyrB. Kinetic experiments were performed to determine the time needed for binding of Novo-TRX and GyrB to reach equilibrium. Equal volumes of 2× Novo-TRX were mixed with 2× GyrB to result in a final concentration of 40 nM Novo-TRX and 25, 50, or 100 nM GyrB, and the polarization was measured every 30 s for 70 cycles. The fluorescence polarization (FP) signal (mP) and the error bars represent the data from 4 replicates. To measure the dissociation of Novo-TRX, 125-fold unlabeled novobiocin was added to the Novo-TRX/GyrB samples, resulting in a final concentration of 10 μM. The change in FP was measured every 30 s for 70 cycles. The average FP signal (mP) for 4 replicates was determined, and the error bars represent the standard deviation.