FIG. 2.
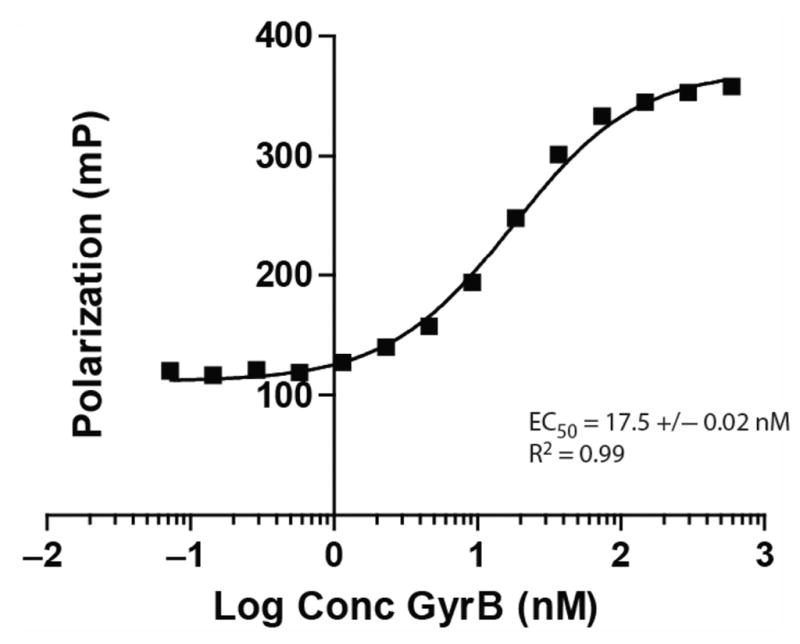
Saturation binding experiments for the interaction of Novo-TRX with GyrB. The homogeneous binding of Novo-TRX to GyrB was measured under equilibrium conditions to determine the amount of GyrB needed to saturate binding. Increasing concentrations (0–2500 nM) of GyrB were incubated with 40 nM Novo-TRX and incubated for 1 h before the binding was measured. The average fluorescence polarization (FP) signal (mP) for 4 replicates was determined, and the error bars represent the standard deviation. To determine the EC50, the data were fit using a sigmoidal dose-response equation.
