Abstract
Background
Solid evidence links schizophrenia (SZ) susceptibility to neurodevelopmental processes involving tyrosine phosphorylation-mediated signaling. Mouse studies implicate the Ptpra gene, encoding protein tyrosine phosphatase RPTPα, in the control of radial neuronal migration, cortical cytoarchitecture, and oligodendrocyte differentiation. The human gene encoding RPTPα, PTPRA, maps to a chromosomal region (20p13) associated with susceptibility to psychotic illness.
Methods
We characterized neurobehavioral parameters, as well as gene expression in the central nervous system, of mice with a null mutation in the Ptpra gene. We searched for genetic association between polymorphisms in PTPRA and schizophrenia risk (2 independent cohorts; total of 1420 cases and 1377 controls), and we monitored PTPRA expression in prefrontal dorsolateral cortex of SZ patients (35 cases, 2 control groups of 35 cases)
Results
We find that Ptpra−/− mice reproduce neurobehavioral endophenotypes of human SZ: sensitization to metamphetamine-induced hyperactivity, defective sensorimotor gating, and defective habituation to a startle response. Ptpra loss of function also leads to reduced expression of multiple myelination genes, mimicking the hypomyelination-associated changes in gene expression observed in post mortem patient brains. We further report that a polymorphism at the PTPRA locus is genetically associated with SZ, and that PTPRA mRNA levels are reduced in post mortem dorsolateral prefrontal cortex of subjects with SZ.
Conclusion
The implication of this well-studied signaling protein in SZ risk and endophenotype manifestation provides novel entry points into the etiopathology of this disease.
Keywords: schizophrenia, tyrosine phosphatase, myelination, mouse model, RPTPα, PTPRA
Introduction
Schizophrenia (SZ, MIM #181500) is diagnosed by the joint appearance of positive (hallucinations, delusions), negative (disturbed affective and social functioning), and cognitive symptoms. Initial hypotheses about the pathophysiological mechanism derive from pharmacological observations: blocking D2 receptors can alleviate positive symptoms, and NMDA-R antagonists can mimic disease symptoms, which provides support for the dopaminergic and glutamatergic hypotheses. Imaging and post mortem analyses reveal that SZ is accompanied by neuropathological abnormalities, including decreased myelin content, atypical neuronal cytoarchitecture, and altered laminar organization, suggesting abnormalities in neural development (1). Gene expression studies indicate abnormalities in myelination (2).
The disease has a significant genetic basis (3). Non-affected kin can display quantifiable neurobehavioral abnormalities, perhaps reflecting manifestation of a subset of genetic predispositions. The identification of SZ-associated genes is starting to provide insights into disease etiology, by implicating molecular signaling pathways. One of the first and most reproducible instances of genetic association with SZ is neuregulin 1 (NRG1), whose product, signaling via ERBB receptor tyrosine kinases, modulates oligodendrocyte development, neuronal migration and differentiation, and glutamatergic and GABA-ergic neurotransmission (4–8). Two other genes in the NRG1 pathway, ERBB4 encoding a tyrosine kinase receptor for NRG1, and PTPRZ1 encoding an ERBB4-associated protein tyrosine phosphatase in the oligodendrocyte lineage, are also genetically associated with SZ (9–11). NRG1 signaling may be functionally linked to NMDA-R modulation (11, 12), perhaps via phosphorylation of the latter by the Src-family tyrosine kinases Fyn (13) and c-Src (14). Among other predisposition genes for SZ are cell adhesion molecules such as CHL1 and NCAM (15–21), the signaling activities of which also rely on Src-family kinases (SFKs).
Receptor protein tyrosine phosphatase RPTPα (encoded by human PTPRA and mouse Ptpra) is a physically associated signaling subunit of CHL1 and NCAM, through its well-documented role in regulating Fyn (22–24). RPTPα can also modulate SFKs downstream of EGF-receptor (ERBB1) activation (25). Ptpra is abundantly expressed in the developing central nervous system (CNS), and remains highly expressed in the adult (26, 27). In mice, loss of Ptpra function is associated with neurodevelopmental defects in peripheral myelination (28), oligodendrocyte differentiation and myelin basic protein (MBP) expression (29), radial cortical migration (27), mis-orientation of apical dendrites of deep layer pyramidal neurons (24), reduced NMDA-R phosphorylation, and impairments in synaptic plasticity and short-term memory (27, 30, 31); many of these effects reflect a function of RPTPα in regulating SFKs (24, 25, 29, 31–33). Interestingly, PTPRA maps to a chromosomal region (20p13) that has been linked to SZ (34, 35).
Given the multifold involvement of RPTPα in neurodevelopmental and signaling pathways associated with SZ, we set out to explore whether loss of Ptpra function in mice engendered neurobehavioral abnormalities or gene expression signatures relevant to SZ. Positive findings led us to pursue association between polymorphisms at the PTPRA locus and disease risk, and changes in PTPRA expression in dorsolateral prefrontal cortex of patients.
MATERIALS AND METHODS
Full details of all procedures can be found in the Supplement.
Mice
Generation of RPTPα-deficient (Ptpra−/−) mice has been described (33). The allele was backcrossed 10 times (N=10) with C57Bl/6J mice. Control (wt) mice were generated by intercross of Ptpra+/− heterozygotes.
Mouse motor and neurobehavioral testing
Spontaneous exploratory locomotor activity and drug-induced hyperactivity were generally assessed as in (36), and prepulse inhibition and acute startle responses as in (37).
Gene expression analysis
RNA was extracted from mouse whole brain and human dorsolateral prefrontal cortex and subject to qPCR analysis.
Genetic association
This was performed essentially as in (38), followed by inclusion of a second independent cohort.
RESULTS
Ptpra−/− mice display enhanced psychostimulant-induced hyperactivity, deficient sensorimotor gating, and failure to habituate to a startle response
Dissection of multifactorial diseases is helped by the identification of genetically-based quantitative non-apparent “endophenotypes” that are proximal consequences of genetic predisposition, but precede or are not necessarily accompanied by manifestation of the disease itself. This reductionist approach is particularly relevant to the dissection of psychiatric disease, and to its animal modeling (39).
RPTPα participates in several processes implicated in pharmacological and neurodevelopmental descriptions of schizophrenia, and Ptpra−/− mice manifest neuropathological abnormalities reminiscent of those reported in patients. To determine whether loss-of-function of mouse Ptpra results in behavioral and neuropsychological abnormalities associated with SZ, we focused on three models: Locomotor response to psychostimulants, pre-pulse inhibition (PPI) as a measure of sensorimotor gating, and the watermaze test for spatial memory.
The studies were performed on a previously described Ptpra null allele (27). We first assessed sensorimotor capabilities to exclude the possibility of compounding effects (Table S1 in the Supplement). Latency to fall off a beam or from an accelerating rotarod revealed no obvious abnormality in general sensorimotor capability of Ptpra−/− mice (beamwalk: F(1,33)=0, p=1 and F(1,33) = 0.298, p=0.589 respectively). Spontaneous exploratory locomotor activity was also unaffected by Ptpra allelic status (F(1,33)=1.983, p=0.169). We concluded that Ptpra loss of function (LOF) does not engender sensorimotor abnormalities that would affect the subsequent analyses.
We subsequently asked whether Ptpra LOF altered the locomotor response to the psychostimulant methamphetamine (MAMPH), a pharmacological model inspired by the dopaminergic hypothesis of SZ (40). We found locomotor activity after acute MAMPH administration (2 mg/kg) to be significantly higher in Ptpra−/− mice than in controls (main effect of genotype F(1,31)=5.753, p=0.023; drug treatment: F(1,31)=72.386, p<0.001; genotype × drug treatment: F(1,31)=8.797, p=0.006; post hoc multiple comparisons: MAMPH (WT) vs. MAMPH (Ptpra−/−): p<0.001) (Fig. 1A).
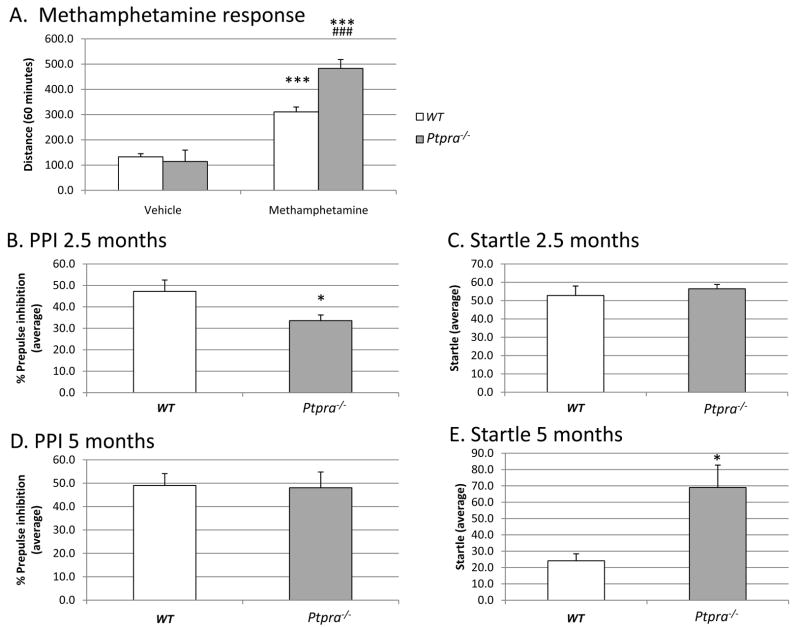
Figure 1. Metamphetamine sensitivity, pre-pulse inhibition (PPI), and startle response in Wt and Ptpra−/− mice.
1A. Influence of genotype on locomotor response to metamphetamine challenge. Methamphetamine resulted in pronounced hyperactivity in both genotypes (p<0.001 vs. veh (wt) and veh (Ptpra−/−) respectively). However, the locomotor response to methamphetamine was exaggerated in Ptpra−/− as compared to WT mice (p<0.001, MAMPH (wt) vs. MAMPH (Ptpra−/−)). The study was run as a within-subject design, where each individual mouse served as its own control by injecting them with either vehicle, MAMPH (2 mg/kg) or MK801 (0.2 mg/kg, data not shown, see main text) in a semi-randomized order ensuring representation of all treatment groups on each test day over 3 days. Compounds were dosed i.p. immediately before test start, n=7–8 (Ptpra−/−) and 8–9 (WT). The animals were 3.5 months old at time of testing. Data are represented as mean distance travelled (± S.E.M) over 60 minutes. Statistical evaluation was performed by applying two-way ANOVA with genotype and drug as factors followed by Fishers LSD test for multiple comparisons. (***: p<0.001 vs. Veh (Ptpra−/−) and veh (WT) respectively)(###: p<0.001 vs. WT-methamphetamine).
1B. Effect of genotype on prepulse inhibition of the acoustic startle response at 2.5 months of age. Ptpra gene disruption leads to reduced prepulse inhibition of the acoustic startle response as compared to wt mice at the age of 2.5 months (p<0.05). Data from 4 different prepulse intensities (pp. 4, pp 8, pp 16 and pp 24) are collapsed and expressed as mean ± S.E.M, n=7 (Ptpra−/−) and 8 (WT). Statistical evaluation was performed by applying two-way ANOVA with genotype and sex as factors (*: p<0.05 vs. WT).
1C. Effect of genotype on acoustic startle response at 2.5 months of age. No effect of Ptpra gene disruption is seen on the startle response to a 120 dB noise burst at the age of 2.5 months of age as compared to wt mice (p>0.05). Data are expressed as mean ± S.E.M, n=7 (Ptpra−/−) and 8 (WT). Statistical evaluation was performed by applying two-way ANOVA with genotype and sex as factors.
1D. Effect of genotype on prepulse inhibition of the acoustic startle response at 5 months of age. At 5 months of age the reduction of prepulse inhibition noted at 2.5 months of age was no longer evident in Ptpra−/− mice (p>0.05 vs. WT) indicating a critical time period for manifestation of this phenotype. Data from 4 different prepulse intensities (pp. 4, pp 8, pp 16 and pp 24) are collapsed and expressed as mean ± S.E.M, n=7 for both genotypes. Statistical evaluation was performed by applying two-way ANOVA with genotype and sex as factors.
1E. Effect of genotype on acoustic startle response at 5 months of age. At 5 months of age, Ptpra−/− mice displayed an increased startle response to a 120 dB noise burst as compared to wildtype mice (p=0.013). This difference is due to a significantly reduced startle response in wildtype mice at the age of 5 months as compared to 2.5 months (p=0.014). This habituated response to a startle inducing stimulus is not evident in Ptpra−/− mice, since the startle response at 5 months of age is similar to that at 2.5 months of age (p=0.914). Data are expressed as mean ± S.E.M, n=7 (Ptpra−/− and WT). Intergroup comparisons were performed by applying two-way ANOVA with genotype and sex as factors. Intragroup comparisons were performed by applying one way repeated measure ANOVA with age and genotype as factors. (*: p<0.05 vs. WT)
Given the functional association of RPTPα with NMDA-R (27, 30, 31), we also investigated the effect of administration of the non-competitive NMDA-R antagonist MK-801, known for its ability to induce psychotic symptoms in healthy humans. However, at the dose used (0.2 mg/kg), MK-801 did not significantly increase locomotor activity in WT mice (not shown).
Secondly, we assessed prepulse inhibition (PPI) of the startle response, a suggested SZ endophenotype. PPI denotes attenuation of a startle motor response to a sensory (acoustic) stimulus when the latter is immediately (<500 msec) preceded by a milder stimulus. Used as an operational measure of sensorimotor gating, PPI is impaired in SZ individuals and their unaffected relatives, and antipsychotics can reverse impairment of PPI in experimental models (41). It constitutes a pre-attentive process akin to a reflex response. We tested the mice on two occasions (2.5 months apart) to determine: (i) if any PPI abnormality persisted; and (ii) whether normal habituation to the startle response occurred, as impaired habituation is a hallmark of schizophrenia (42). Such a longitudinal design is rarely applied to KO mice, partly due to difficulties in the use of test batteries in rodents (43, 44).
At the initial age of analysis (2.5 months), we observed no difference in startle response between Ptpra−/− and WT mice (Fig. 1C) (F(1,14)= 0.332, p=0.576). By contrast, Ptpra−/− mice manifested a significant reduction in PPI (Fig. 1B) (F(1,14)=6.006, p=0.032). This genotype-dependent difference in PPI disappeared at a more advanced age (F(1,13)=0.034, p=0.857) (5 months; Fig. 1D), suggesting a critical time-period for manifestation of this abnormal phenotype. Strikingly however, at the more advanced age, the typical habituation (reduced response) to the acoustic startle stimulus alone observed in WT animals (startle at 2.5 months as compared to 5 months: F(1,14)=11.797, p=0.014) did not occur in Ptpra−/− mice (startle at 2.5 months in comparison to 5 months: F(1,13)=0.013, p=0.914), leading to a significant difference in acoustic startle response during this re-testing at 5 months (Fig. 1E).
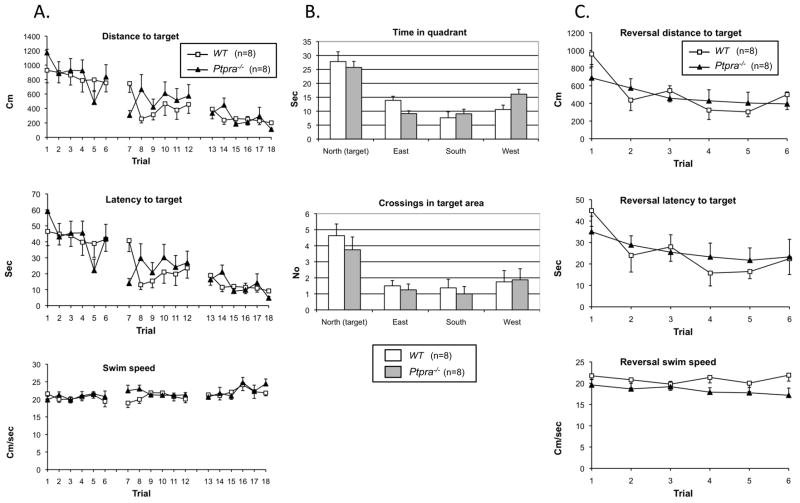
Finally, we subjected Ptpra−/− mice to a water maze test, a hippocampal-dependent model of spatial memory. Impaired hippocampal-based function in SZ is well-documented (45). Detailed analysis revealed no genotype differences in place finding (swim distance and latency to target; Fig 2A), nor in the probe test (recall of spatial position of the platform; Fig 2B), or reversal learning (Fig. 2C).
Figure 2. Morris water maze analysis of Wt and Ptpra−/− mice.
2A. Training: swim distance to target, escape latency and swim speed. Both wildtype (n=8) and Ptpra−/− (n=8) used shorter distances to locate the hidden platform over trials. No effect of genotype or genotype × trial interaction on distance to platform, escape latency or swim speed was found. One-way RM ANOVA wildtype: F(17,119)=4,982; p<0.001 and Ptpra−/−: F(17,119)=5.477; p<0.001). Two-way RM ANOVA found no effect of genotype for distance (F(1,238)=0.803; p=0.385) and no genotype × trial interaction (F(17,238)=1.231; p=0.241).
2B. Probe test: time in quadrants and crossings in platform area. Both genotypes spent significantly longer time in the northern quadrant where the platform used to be located than in other quadrants, and had more crossings in the area previously occupied by the platform than in areas of identical size and position in the other quadrants.
Time spent in northern quadrant compared with other quadrants (P=0.006 or less two-way RM ANOVA, Fisher LSD post hoc); no effect of genotype (F(1,42)=1.340; p=0.266) or genotype × quadrant interaction (F(3,42)=1.801; p=0.162).
Number of crossings in the area where the platform was during training (D=9 cm) compared with crossings in areas of equal size and position in the other quadrants (F(3,42)=9.199; p<0.001); no effect of genotype (F(1,42)=1.317; p=0.27) or genotype × quadrant interaction (F(3,42)=0.203; p=0.894). Post hoc analysis revealed significantly more crossings in the target area for both groups (p=0.047 or less) compared to crossings in areas equal in size and position in the other quadrants.
2C. Reversal learning: swim distance to target, escape latency and swim speed during reversal learning. Two-way RM ANOVA on distance to target showed significant effect of trial (F(5,70)=3.383; p=0.009) but no effect of genotype (F(1,70)=0.0591; p=0.811) or genotype × trial interaction (F(5,70)=0.757; p=0.584). No effect of genotype or genotype × trial interaction was found on escape latency or swim speed.
Loss of Ptpra function leads to reduced CNS levels of myelin markers and SZ-associated genes
Increased metamphetamine sensitivity, impaired PPI, and failure to habituate to a startle response are commonly accepted indicators for modeling SZ-associated states in mice. To assess whether the relevance of Ptpra−/− mice as a model for SZ-associated abnormalities extends beyond neuropsychological parameters, we started assessing SZ-associated gene expression markers. Imaging analysis, post mortem brain studies, genetic association studies, and gene expression studies reveal that abnormal oligodendroglial function and myelination are commonly associated with schizophrenia (2, 46–49). One of the major targets of RPTPα, the Src family kinase Fyn, plays important roles in myelination (50–53). A transient defect in peripheral myelination has been documented in the strain of Ptpra−/− mice studied here (28); an independently generated Ptpra−/− strain was recently reported to display impaired oligodendrocyte differentiation in vitro, and reduced MBP immunostaining in vivo (29). We therefore investigated the expression of myelin related genes in the brains of our strain of Ptpra−/− mice.
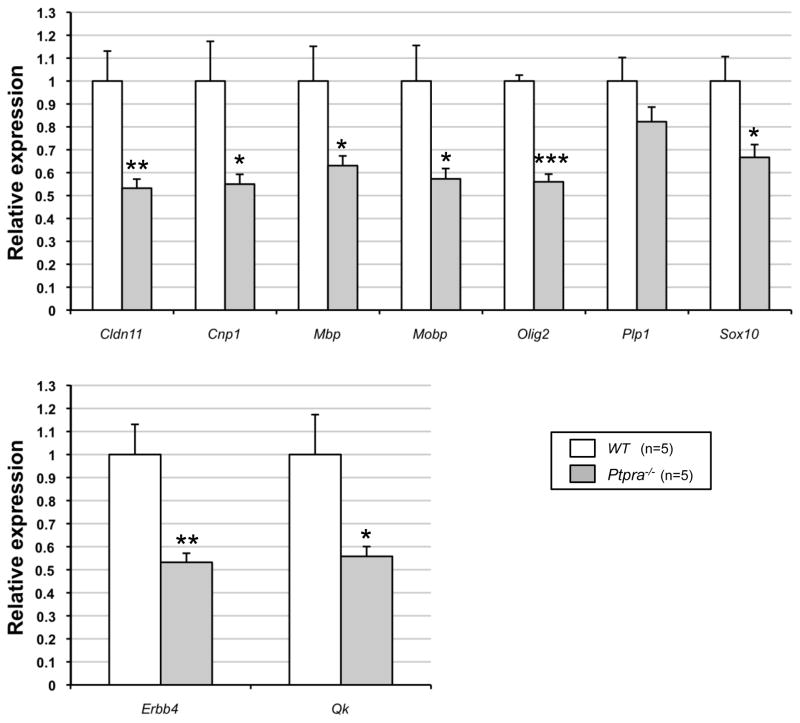
We found that mRNA levels of 8/9 tested oligodendrocyte lineage marker genes were significantly (p<0.05 to p<0.001) reduced (range 53–67 %) in Ptpra−/− mice (Fig. 3). This phenomenon applied not only to an oligodendrocyte marker (myelin basic protein, MBP), but also to genes that are functionally involved in oligodendrocyte differentiation (e.g. Sox10, Qk), and oligodendrocyte lineage genes whose reduced expression in human SZ brain is well-documented (e.g. Cnp1, Cldn11, Qk) (48), or that are genetically associated with SZ (e.g. Erbb4 (9) and Qk (54)).
Figure 3. Reduced expression of oligodendrocyte- and myelin-related (OMR) gene expression in total brain of Ptpra−/− mice.
5 month old animals (5 males/genotype) were analyzed by qPCR. Four endogenous control genes (Actb, B2m, Gusb, and Ppia) were used for normalization.
(*: p < 0.05; **: p < 0.01; ***: p < 0.001 vs. wt)
A polymorphism in human PTPRA demonstrates close genetic association with schizophrenia susceptibility
Our finding that ablation of mouse Ptpra mimics neuropsychological and gene expression abnormalities associated with SZ prompted us to pursue a genetic link between human PTPRA and SZ risk. PTPRA maps to 20p13, identified as a susceptibility locus by low-resolution linkage studies in two different human groups (34, 35). We pursued more detailed SNP fine-mapping analysis on a third population to search for evidence for closer association between SZ and PTPRA.
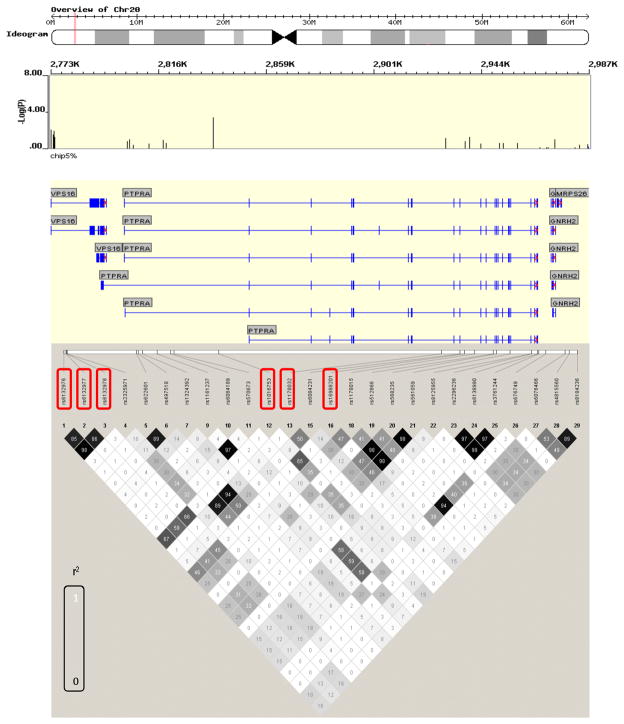
In the first stage, 560 cases and 548 controls were genotyped using the GeneChip® Human Mapping 5.0 Array (Affymetrix). Out of 21 SNPs genotyped across the PTPRA locus, six yielded nominally significant association with SZ (rs6132976, rs6132977, rs6132978, rs1016753, rs1178032 and rs16988201) (best uncorrected p=0.002). To confirm this association, we performed a replication using an independent sample comprised of 850 cases and 829 controls. Based on the linkage disequilibrium (LD) pattern from the first stage analysis, three SNPs (rs1016753, rs1178032, and rs16988201) were selected (rs6132976, rs6132977 and rs6132978 were represented by rs1016753; Figure 4). Analysis of imputation (Table S2 in the Supplement) and LD pattern within the PTPRA locus suggested that the SNPs selected for follow up capture all ungenotyped SNPs which increase the risk of developing schizophrenia. In the replication, only rs1016753 showed significant association (p=0.04), with the same direction of association (Breslow-Day P=0.218). Pooled analysis of 1st and 2nd (1420 cases, 1377 controls) showed highly significant association of this SNP with schizophrenia (p = 0.0008) (Table S3 in the Supplement).
Figure 4. Genetic association of SNPs around and in the PTPRA gene with schizophrenia.
Red boxes indicate nominally associated SNPs in the 1st stage analysis (GWAS screening sample). r2 is the correlation coefficient between the two loci. The numbers are correlation coefficients calculated based on the GWAS sample.
Reduced PTPRA expression levels in dorsolateral and prefrontal cortex from schizophrenia patients
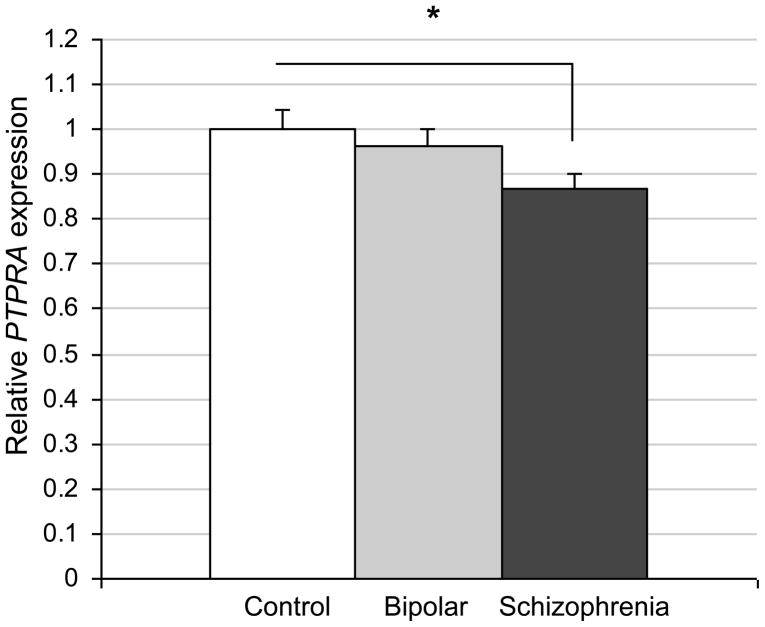
As an independent approach to explore a possible involvement of PTPRA in schizophrenia, we examined its expression level in post mortem samples from patients, as compared to healthy controls and to sufferers from bipolar disorder (35 each). qPCR analysis (Fig. 5) showed that PTPRA expression was significantly reduced in dorsolateral prefrontal cortex from SZ patients (13 % decrease; p=0.018), with trend level reductions in samples from patients with bipolar disorder (p=0.078).
Figure 5. Reduced PTPRA expression levels in dorsolateral prefrontal cortex specimens from schizophrenia patients.
35 patient samples for each category (healthy controls, patients with schizophrenia, and patients with bipolar disorder) were analyzed by qPCR. Four endogenous control genes (ACTB, GAPD, GUSB, and PPIA) were used for normalization (*: p < 0.05 vs. control).
DISCUSSION
This study was prompted by implications of Ptpra in developmental processes linked to schizophrenia (neuronal migration, myelination); by RPTPα acting as a signaling subunit for cell adhesion molecules (NCAM and CHL1) whose genes have been related to SZ risk; and by the mapping of a schizophrenia locus close to PTPRA. The avenues we explored provide independent lines of convergent evidence linking RPTPα to SZ: typical changes in neuropsychological parameters in RPTPα-deficient mice; association of the human gene with disease risk; and reduced cortical PTPRA expression in SZ patients.
Behavioral characteristics of Ptpra−/− mice relevant to SZ
We demonstrate that Ptpra LOF is associated with enhanced methamphetamine responsiveness (Fig. 1A), defective sensorimotor gating as measured by PPI (Fig. 1B), and failure to habituate to a startle response (Fig. 1E). All these endpoints implicate a schizophrenia-like profile based on current clinical knowledge. The deficits could not be accounted for by obvious sensorimotor deficits, since Ptpra−/− mice did not display differences in motility, rotarod and beam walk tests, or initial startle response.
The enhanced response of Ptpra−/− mice to MAMPH suggests an augmented dopaminergic system (40). In a previous study (55), Skelton et al. failed to detect an altered amphetamine response in a different Ptpra−/− strain; this negative result may reflect a different dosing regime (we used 2 mg/kg whereas Skelton et al. used 1 mg/kg), or, more likely, a less uniform genetic background. We backcrossed our Ptpra−/− allele ten times into inbred C57Bl/6J. By contrast, the founder animals of Skelton et al. were crossed into outbred Black Swiss mice (32, 55); the ensuing higher genetic heterogeneity may have made the change in MAMPH responsiveness associated with loss of Ptpra function difficult to detect. In the absence of studies on the effect of Ptpra ablation on dopamine receptor expression, agonist binding, or activity, it seems premature to speculate about the mechanism of the MAMPH effect. Strikingly, haloperidol-induced catalepsy requires the Fyn gene, and this drug activates the FYN kinase in striatum (56). As Fyn is a well-established RPTPα target (25, 29, 32, 33), defective Fyn activation in absence of RPTPα may fail to inhibit striatal dopamine signaling.
Contrasting with the increased MAMPH responsiveness in Ptpra−/− mice, we observed no effect of Ptpra status on sensitivity to the glutamate antagonist MK-801. Whilst somewhat surprising given links between RPTPα and NMDA-R (30, 31), our negative finding may merely be a function of the selected dose (0.2 mg/kg), since no hyperactive response was seen in the control mice either. In our hands, 0.2 mg/kg MK-801 reproducibly induces hyperactivity in outbred NMRI mice (not shown). Therefore, pending further dose exploration in the Ptpra−/− mice, we suggest the failure to detect changes in MK-801 responsiveness is equivocal.
2.5 months old Ptpra−/− mice show a pronounced PPI deficit (Fig. 1B). “Inhibitory failure” revealed by defective PPI is considered a correlate of defects in acute attention and gating associated with psychiatric diseases, including schizophrenia. As an endophenotype, PPI is decreased in non-affected relatives of SZ patients, suggesting it may be a proximal indicator of genetic susceptibility (39). Interestingly, the PPI deficit in Ptpra−/− mice did not persist when the same mice were re-tested at 5 months of age (Fig. 1D). This restriction of the deficit to early adulthood suggests involvement of compensatory changes with aging. Since knockout of Ptpra alone cannot sustain this phenotype, Ptpra−/− mice may provide a point-of-entry to identify genetic or environmental parameters that will specify PPI extinction or exacerbation. Such studies may provide insights into factors and processes that determine SZ prognosis.
The startle response in mice displays plasticity not only in terms of gating, but also in terms of habituation. Whereas Ptpra−/− and WT mice had an equivalent startle response at 2.5 months (Fig. 1C), we observed habituation by 5 months in WT but not Ptpra−/− mice (Fig. 1E). This finding is interesting since schizophrenics also have a deficit in habituation, for example, the eye blink reflex in response to auditory stimuli (57). A deficit in pre-attentive inhibitory mechanisms to extraneous information is thought to underlie an altered habituation response in schizophrenia (42).
We found no deficit in Morris water maze acquisition or memory in Ptpra−/− mice. Here again, our findings seem to differ from Skelton et al. (55) who did report such a deficit. The different genetic background may again constitute a first possible explanation. In addition, Skelton et al. report water maze defects only under very particular conditions (platform in the NE, but not in the SW quadrant); this interaction between spatial environment and genotype may primarily reflect subtle effects of Ptpra status on sensitivity to spatial cues or on orientation skills. Interestingly, Ptpra−/− mice display defective learning in a radial arm water maze test (27), which may reflect the higher sensitivity, and/or increased requirement for short-term and working memory of this assay. Taken together, the present findings and those of Skelton et al. (55) and Petrone et al. (27) indicate that the hippocampal system is not overly impacted by Ptpra LOF, in spite of the architectural abnormalities in hippocampus resulting from radial migratory dysfunction in Ptpra−/− mice (27).
Genetic association of PTPRA with SZ
KO studies can be confounded by flanking markers from back- or outcrossing, or by inadvertent consequences of genome manipulation unrelated to changes in Ptpra function (e.g. altered expression of known or unknown flanking genes). A strong case can be made for a direct link between Ptpra and the observed phenotypes. Two independent Ptpra−/− mice both reveal effects of Ptpra knockdown on NMDA-R phosphorylation (27, 31); an electrophysiological study shows rescue of the NMDA-R gating defect in Ptpra−/− cells by RPTPα expression, and mimicking of the defect by antibodies against RPTPα (30). The two lines also show similar effects on SFK-dependent pathways, which can also be rescued by RPTPα expression (33, 58, 59), or mimicked by RPTPα knockdown (29, 60, 61). Indeed, many Ptpra−/− phenotypes can be clearly linked to deregulation of the 2 best-established RPTPα substrates, the tyrosine kinases c-Src and Fyn (e.g. (22, 62)). Our finding impaired oligodendrocyte marker expression in Ptpra−/− mice is again consistent with studies using an independent Ptpra LOF allele and different assays (29), and with Fyn dysfunction (63).
The collective mouse evidence thus makes PTPRA a valid candidate for follow-up study in humans. Accordingly, we report highly significant association of a SNP in PTPRA with schizophrenia in a Japanese population. The sample size (~ 2600) is enough to detect mild to moderate effects of SNPs, and the evidence of PTPRA association is robust, since the two-stage analysis reduces the potential for type I error. Based on LD analysis in the 1st stage, we selected rs1016753 as a representative SNP for rs6132976, rs6132977 and rs6132978. Therefore, the association of rs1016753 might reflect possible association of these or other linked SNPs. Since the LD structure of PTPRA is relatively loose, we cannot narrow down the associated region to identify the “true” SNPs. Unbiased genome-wide association studies searching for genetic SZ risk determinants have failed to implicate the PTPRA locus; our focus on a particular population may have lowered the detection threshold, for environmental or genetic reasons.
It remains premature to speculate about the relevance of rs1016753 or linked SNPs for PTPRA function. PTPRA expression and the choice among alternative splicing events can be surveyed. Based on exon array data (not shown), we performed QPCR on immortalized lymphoblastoid cell lines derived from 48 participants in the association study (43 CC carriers, 5 CG carriers), using primers directed against exons specific to each of the 3 PTPRA transcripts described by NCBI. This revealed significantly increased expression of the NM_080840.2 transcript (as defined by its “exon 1” with physical position Chr20: 2,802,142–2,802,406 based on NCBI B36 assembly), but not of the NM_002836.3 and NM_080841.2 transcripts, in CG as compared to CC carriers (Table S4 in the Supplement). Unfortunately, we were unable to perform a similar analysis on the human brain samples used for figure 5, due to the low minor allele frequency in this cohort. At the protein level, the effect of rs1016753 allelic status and altered NM_080840.2 expression on the balance between two RPTPα isoforms with known differences in biological activity (64, 65) can also be a subject for further inquiry.
Molecular pathways involving PTPRA relevant to SZ
RPTPα is a signaling mediator for surface molecules that are themselves devoid of catalytic activity, most prominently integrins and canonical CAMs. Among the latter, there are reports for cis association and signaling functions of RPTPα in the context of TAG-1 (50), contactin (66), NCAM (22), and the CHL1/NB-3 complex (24).
Adult Ptpra−/− mice show a decrease of oligodendrocyte lineage gene expression. Further in vivo studies will be needed to establish whether this effect is primary or degenerative, and whether it is autonomous to the OLG lineage. The Pallen group recently reported decreased MBP protein levels in the brain of P18 Ptpra−/− mice, and provided strong evidence for a lineage-autonomous role for RPTPα in oligodendrocyte differentiation in vitro, with deficient Fyn activation as a plausible mechanism (29, 53). Interestingly, oligodendrocyte Fyn integrates signaling in a complex between contactin-1 and integrins (67), i.e. between members of 2 classes of cell surface molecules that rely on RPTPα for signal transduction (58, 66). Abnormalities in oligodendrocyte function are a robust biological marker of human schizophrenia (48, 49), but elucidation of links between myelination and the disease still remains more a matter of speculation than of hypothesis testing. A broad question is how to link the white matter abnormalities in patients to the as yet more clinically relevant pharmacological evidence of neurotransmitter pathway dysfunction. The Ptpra−/− model may play a valuable role in exploring this issue. More specifically, manipulation of the Ptpra gene will be useful to explore to what extent the neurobehavioral abnormalities result from loss of Ptpra function in the neuronal or oligodendroglial lineage, and whether or how Ptpra dysfunction in one lineage may impact other lineages, and neurotransmitter systems.
CAMs are linked to NMDA neurotransmission, dysfunction of which is also linked to SZ. Long-term potentiation (LTP) at CA3-CA1 excitatory synapses is reduced in Chl1−/− mice (68) and in a hippocampal-specific NCAM knockout (69); NMDA-mediated behavioral alterations have also been observed in these mice (69, 70). A recent study links NCAM poly-sialylation to NMDA-R signaling (71). Absence of the NCAM isoform NCAM180 leads to increased lateral ventricle size, one of the most reliable morphological features in brains of schizophrenics, and is often accompanied by cognitive impairments (70). Association studies implicate NCAM and CHL1 in human SZ risk, and LOF of the corresponding genes in mice engenders intriguing phenotypic overlaps with Ptpra LOF, in terms of cortical radial migration (72), dendrite orientation (24), impaired LTP (69), and impaired sensorimotor gating/PPI (73). Thus, phenotypes observed in Ptpra−/− mice could be mediated by the effect of RPTPα on these molecules.
The best characterized substrate and effector for RPTPα in NCAM- and CHL1/NB-3 signaling complexes is the SFK Fyn. That RPTPα is a net activator of Fyn kinase activity (32, 33) would be consistent with phenotypic overlap between LOF in either gene. Like Ptpra−/− mice (27, 29), Fyn−/− mice exhibit abnormal long-term potentiation, spatial learning, radial migration, myelination (62, 74), and myelin gene expression (51). Genetic association of FYN with SZ was reported as absent (75), although there are positive data about prefrontal function in patients (76). Interestingly, Fyn is required for haloperidol signaling in striatal neurons (56), and platelets from SZ patients show decreased expression and altered FYN splicing (77). Fyn also phosphorylates NMDA-R subunits (52). Phosphorylation of NMDA-R subunits is reduced in Ptpra−/− mice, and RPTPα associates with and controls gating of NMDA-R (27, 30, 31). Thus, reduced RPTPα function could contribute to a schizophrenic phenotype through impairment of Fyn activity.
Taken together, one can envision a SZ-relevant pathway as NCAM/CHL1-NB3 → RPTPα → Fyn → NMDA-R. However, this is mainly a highly speculative working hypothesis. Not only are the links between Fyn and SZ relatively tenuous, there are also important phenotypic differences between Fyn−/− and Ptpra−/− mice (e.g. in hippocampal structure), RPTPα can act on other SZ-relevant SFKs (including c-Src (14)), and RPTPα directs SFKs towards only a subset of their substrates (25).
Our findings also warrant consideration of cross-talk of RPTPα with the NRG1 - ERBB4 pathway. RPTPα can affect ERBB1 signaling (25, 78), and we find that Ptpra ablation results in reduced Erbb4 expression (Figure 3). NRG1/ERBB4 signaling suppresses upregulation of NMDA-R by c-Src (14). Nrg1+/− mice show reduced Fyn/Pyk2-mediated phosphorylation of Y1472 in the NR2B subunit of NMDA-R, that can be rescued by the antipsychotic clozapine (13); it remains to be seen whether clozapine can reverse the reduced phosphorylation of Fyn and NR2B and the abnormal behavior in Ptpra−/− mice.
The convergent evidence reported here linking RPTPα to schizophrenia can allow enunciation of novel hypotheses and open up avenues for modeling and dissection of the disease mechanism that may yield clues for therapeutic exploration.
Supplementary Material
Acknowledgments
Research funded by the Lundbeck Foundation and by Vera og Carl Johan Michaelsens legat (JS). TS and JDB acknowledge the support of the NIMH through a Conte Center grant MH066392 and the Stanley Foundation. TS was supported by the New York State spinal cord injury research program. NT appreciates the Mitsubishi Pharma Research Foundation for their support. BA and NO acknowledge the support of the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Core Research for Evolutional Science and Technology.
JS gratefully acknowledges Kjeld Møllgård and Niels-Henrik Holstein-Rathlou at the University of Copenhagen for support. We thank Bente Møller and Tanya Yankelevich for genotyping. The invaluable support of Lisa Gasperini, Giuseppe Legname, Marco Stebel, Yotis Senis, and Mauro Torti with mouse colony management is also greatly appreciated.
Abbreviations
- CAM
cell adhesion molecule
- GWAS
Genome-wide association study
- LD
linkage disequilibrium
- LOF
loss of function
- MAMPH
Metamphetamine
- MBP
myelin basic protein
- NMDA-R
NMDA-receptor
- OMR
oligodendrocyte- and myelin-related
- PPI
pre-pulse inhibition
- PTP
protein tyrosine phosphatase
- RPTP
receptor protein tyrosine phosphatase
- SFK
Src-family kinase
- SNP
single nucleotide polymorphism
- SZ
schizophrenia
- veh
vehicle
Footnotes
FINANCIAL DISCLOSURES
All authors reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- 2.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- 4.Fischbach GD. NRG1 and synaptic function in the CNS. Neuron. 2007;54:495–497. doi: 10.1016/j.neuron.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 6.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 10.Buxbaum JD, Georgieva L, Young JJ, Plescia C, Kajiwara Y, Jiang Y, et al. Molecular dissection of NRG1-ERBB4 signaling implicates PTPRZ1 as a potential schizophrenia susceptibility gene. Mol Psychiatry. 2008;13:162–172. doi: 10.1038/sj.mp.4001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett M. Positive and negative symptoms in schizophrenia: the NMDA receptor hypofunction hypothesis, neuregulin/ErbB4 and synapse regression. Aust N Z J Psychiatry. 2009;43:711–721. doi: 10.1080/00048670903001943. [DOI] [PubMed] [Google Scholar]
- 12.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 13.Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, et al. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17:470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poltorak M, Khoja I, Hemperly JJ, Williams JR, el-Mallakh R, Freed WJ. Disturbances in cell recognition molecules (N-CAM and L1 antigen) in the CSF of patients with schizophrenia. Exp Neurol. 1995;131:266–272. doi: 10.1016/0014-4886(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 16.Poltorak M, Wright R, Hemperly JJ, Torrey EF, Issa F, Wyatt RJ, et al. Monozygotic twins discordant for schizophrenia are discordant for N-CAM and L1 in CSF. Brain Res. 1997;751:152–154. doi: 10.1016/s0006-8993(97)00023-1. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai K, Migita O, Toru M, Arinami T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Mol Psychiatry. 2002;7:412–415. doi: 10.1038/sj.mp.4000973. [DOI] [PubMed] [Google Scholar]
- 18.Chen QY, Chen Q, Feng GY, Lindpaintner K, Chen Y, Sun X, et al. Case-control association study of the close homologue of L1 (CHL1) gene and schizophrenia in the Chinese population. Schizophr Res. 2005;73:269–274. doi: 10.1016/j.schres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Atz ME, Rollins B, Vawter MP. NCAM1 association study of bipolar disorder and schizophrenia: polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sullivan PF, Keefe RS, Lange LA, Lange EM, Stroup TS, Lieberman J, et al. NCAM1 and neurocognition in schizophrenia. Biol Psychiatry. 2007;61:902–910. doi: 10.1016/j.biopsych.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 21.van Kammen DP, Poltorak M, Kelley ME, Yao JK, Gurklis JA, Peters JL, et al. Further studies of elevated cerebrospinal fluid neuronal cell adhesion molecule in schizophrenia. Biol Psychiatry. 1998;43:680–686. doi: 10.1016/s0006-3223(97)00324-7. [DOI] [PubMed] [Google Scholar]
- 22.Bodrikov V, Leshchyns’ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M. RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation. J Cell Biol. 2005;168:127–139. doi: 10.1083/jcb.200405073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodrikov V, Sytnyk V, Leshchyns’ka I, den Hertog J, Schachner M. NCAM induces CaMKIIalpha-mediated RPTPalpha phosphorylation to enhance its catalytic activity and neurite outgrowth. J Cell Biol. 2008;182:1185–1200. doi: 10.1083/jcb.200803045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye H, Tan YL, Ponniah S, Takeda Y, Wang SQ, Schachner M, et al. Neural recognition molecules CHL1 and NB-3 regulate apical dendrite orientation in the neocortex via PTP alpha. Embo J. 2008;27:188–200. doi: 10.1038/sj.emboj.7601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vacaresse N, Moller B, Danielsen EM, Okada M, Sap J. Activation of c-Src and Fyn kinases by protein-tyrosine phosphatase RPTPalpha is substrate-specific and compatible with lipid raft localization. J Biol Chem. 2008;283:35815–35824. doi: 10.1074/jbc.M807964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.den Hertog J, Overvoorde J, de Laat SW. Expression of receptor protein-tyrosine phosphatase alpha mRNA and protein during mouse embryogenesis. Mech Dev. 1996;58:89–101. doi: 10.1016/s0925-4773(96)00561-8. [DOI] [PubMed] [Google Scholar]
- 27.Petrone A, Battaglia F, Wang C, Dusa A, Su J, Zagzag D, et al. Receptor protein tyrosine phosphatase α is essential for hippocampal neuronal migration and long-term potentiation. EMBO J. 2003;22:4121–4131. doi: 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiran Z, Peretz A, Sines T, Shinder V, Sap J, Attali B, et al. Tyrosine phosphatases epsilon and alpha perform specific and overlapping functions in regulation of voltage-gated potassium channels in Schwann cells. Mol Biol Cell. 2006;17:4330–4342. doi: 10.1091/mbc.E06-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang PS, Wang J, Xiao ZC, Pallen CJ. Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J Biol Chem. 2009;284:33692–33702. doi: 10.1074/jbc.M109.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei G, Xue S, Chery N, Liu Q, Xu J, Kwan CL, et al. Gain control of N-methyl-D-aspartate receptor activity by receptor-like protein tyrosine phosphatase alpha. EMBO J. 2002;21:2977–2989. doi: 10.1093/emboj/cdf292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le HT, Maksumova L, Wang J, Pallen CJ. Reduced NMDA receptor tyrosine phosphorylation in PTPalpha-deficient mouse synaptosomes is accompanied by inhibition of four src family kinases and Pyk2: an upstream role for PTPalpha in NMDA receptor regulation. J Neurochem. 2006;98:1798–1809. doi: 10.1111/j.1471-4159.2006.04075.x. [DOI] [PubMed] [Google Scholar]
- 32.Ponniah S, Wang DZ, Lim KL, Pallen CJ. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases src and Fyn. Curr Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- 33.Su J, Muranjan M, Sap J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- 34.Fanous AH, Neale MC, Webb BT, Straub RE, O’Neill FA, Walsh D, et al. Novel linkage to chromosome 20p using latent classes of psychotic illness in 270 Irish high-density families. Biol Psychiatry. 2008;64:121–127. doi: 10.1016/j.biopsych.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Teltsh O, Kanyas K, Karni O, Levi A, Korner M, Ben-Asher E, et al. Genome-wide linkage scan, fine mapping, and haplotype analysis in a large, inbred, Arab Israeli pedigree suggest a schizophrenia susceptibility locus on chromosome 20p13. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:209–215. doi: 10.1002/ajmg.b.30591. [DOI] [PubMed] [Google Scholar]
- 36.Butini S, Gemma S, Campiani G, Franceschini S, Trotta F, Borriello M, et al. Discovery of a new class of potential multifunctional atypical antipsychotic agents targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors: design, synthesis, and effects on behavior. J Med Chem. 2009;52:151–169. doi: 10.1021/jm800689g. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen JT, Andersen KK, Nielsen EO, Mathiasen L, Mirza NR. Nicotine and clozapine selectively reverse a PCP-induced deficit of PPI in BALB/cByJ but not NMRI mice: comparison with risperidone. Behav Brain Res. 2006;167:118–127. doi: 10.1016/j.bbr.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Ikeda M, Aleksic B, Kinoshita Y, Okochi T, Kawashima K, Kushima I, et al. Genome-Wide Association Study of Schizophrenia in a Japanese Population. Biol Psychiatry. 2011;69:472–478. doi: 10.1016/j.biopsych.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 40.Marcotte ER, Pearson DM, Srivastava LK. Animal models of schizophrenia: a critical review. J Psychiatry Neurosci. 2001;26:395–410. [PMC free article] [PubMed] [Google Scholar]
- 41.Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- 42.Meincke U, Light GA, Geyer MA, Braff DL, Gouzoulis-Mayfrank E. Sensitization and habituation of the acoustic startle reflex in patients with schizophrenia. Psychiatry Res. 2004;126:51–61. doi: 10.1016/j.psychres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 43.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 44.Voikar V, Vasar E, Rauvala H. Behavioral alterations induced by repeated testing in C57BL/6J and 129S2/Sv mice: implications for phenotyping screens. Genes Brain Behav. 2004;3:27–38. doi: 10.1046/j.1601-183x.2003.0044.x. [DOI] [PubMed] [Google Scholar]
- 45.Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res. 2008;14:97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, et al. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 47.Hoistad M, Segal D, Takahashi N, Sakurai T, Buxbaum JD, Hof PR. Linking white and grey matter in schizophrenia: oligodendrocyte and neuron pathology in the prefrontal cortex. Front Neuroanat. 2009;3:9. doi: 10.3389/neuro.05.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martins-de-Souza D. Proteome and transcriptome analysis suggests oligodendrocyte dysfunction in schizophrenia. J Psychiatr Res. 2010;44:149–156. doi: 10.1016/j.jpsychires.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Prog Neurobiol. 2011;93:13–24. doi: 10.1016/j.pneurobio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- 51.Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, et al. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu Z, Ku L, Chen Y, Feng Y. Developmental abnormalities of myelin basic protein expression in fyn knock-out brain reveal a role of Fyn in posttranscriptional regulation. J Biol Chem. 2005;280:389–395. doi: 10.1074/jbc.M405973200. [DOI] [PubMed] [Google Scholar]
- 53.Kramer-Albers EM, White R. From axon-glial signalling to myelination: the integrating role of oligodendroglial Fyn kinase. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-010-0616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aberg K, Saetre P, Lindholm E, Ekholm B, Pettersson U, Adolfsson R, et al. Human QKI, a new candidate gene for schizophrenia involved in myelination. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:84–90. doi: 10.1002/ajmg.b.30243. [DOI] [PubMed] [Google Scholar]
- 55.Skelton MR, Ponniah S, Wang DZ, Doetschman T, Vorhees CV, Pallen CJ. Protein tyrosine phosphatase alpha (PTP alpha) knockout mice show deficits in Morris water maze learning, decreased locomotor activity, and decreases in anxiety. Brain Res. 2003;984:1–10. doi: 10.1016/s0006-8993(03)02839-7. [DOI] [PubMed] [Google Scholar]
- 56.Hattori K, Uchino S, Isosaka T, Maekawa M, Iyo M, Sato T, et al. Fyn is required for haloperidol-induced catalepsy in mice. J Biol Chem. 2006;281:7129–7135. doi: 10.1074/jbc.M511608200. [DOI] [PubMed] [Google Scholar]
- 57.Geyer MA, Braff DL. Habituation of the blink reflex in normals and schizophrenic patients. Psychophysiology. 1982;19:1–6. doi: 10.1111/j.1469-8986.1982.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 58.von Wichert G, Jiang G, Kostic A, De Vos K, Sap J, Sheetz MP. RPTP-alpha acts as a transducer of mechanical force on αv/β3-integrin-cytoskeleton linkages. J Cell Biol. 2003;161:143–153. doi: 10.1083/jcb.200211061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen M, Chen SC, Pallen CJ. Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration. J Biol Chem. 2006;281:11972–11980. doi: 10.1074/jbc.M600561200. [DOI] [PubMed] [Google Scholar]
- 60.Zheng X, Resnick RJ, Shalloway D. Apoptosis of estrogen-receptor negative breast cancer and colon cancer cell lines by PTP alpha and src RNAi. Int J Cancer. 2008;122:1999–2007. doi: 10.1002/ijc.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krndija D, Schmid H, Eismann JL, Lother U, Adler G, Oswald F, et al. Substrate stiffness and the receptor-type tyrosine-protein phosphatase alpha regulate spreading of colon cancer cells through cytoskeletal contractility. Oncogene. 2010;29:2724–2738. doi: 10.1038/onc.2010.25. [DOI] [PubMed] [Google Scholar]
- 62.Yuasa S, Hattori K, Yagi T. Defective neocortical development in Fyn-tyrosine-kinase-deficient mice. Neuroreport. 2004;15:819–822. doi: 10.1097/00001756-200404090-00016. [DOI] [PubMed] [Google Scholar]
- 63.Goto J, Tezuka T, Nakazawa T, Sagara H, Yamamoto T. Loss of Fyn tyrosine kinase on the C57BL/6 genetic background causes hydrocephalus with defects in oligodendrocyte development. Mol Cell Neurosci. 2008;38:203–212. doi: 10.1016/j.mcn.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Kapp K, Siemens J, Weyrich P, Schulz JB, Haring HU, Lammers R. Extracellular domain splice variants of a transforming protein tyrosine phosphatase alpha mutant differentially activate Src-kinase dependent focus formation. Genes Cells. 2007;12:63–73. doi: 10.1111/j.1365-2443.2006.01034.x. [DOI] [PubMed] [Google Scholar]
- 65.Tremper-Wells B, Resnick RJ, Zheng X, Holsinger LJ, Shalloway D. Extracellular domain dependence of PTPalpha transforming activity. Genes Cells. 2010;15:711–724. doi: 10.1111/j.1365-2443.2010.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zeng L, D’Alessandri L, Kalousek MB, Vaughan L, Pallen CJ. Protein tyrosine phosphatase alpha (PTPalpha) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J Cell Biol. 1999;147:707–714. doi: 10.1083/jcb.147.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laursen LS, Chan CW, ffrench-Constant C. An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci. 2009;29:9174–9185. doi: 10.1523/JNEUROSCI.5942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nikonenko AG, Sun M, Lepsveridze E, Apostolova I, Petrova I, Irintchev A, et al. Enhanced perisomatic inhibition and impaired long-term potentiation in the CA1 region of juvenile CHL1-deficient mice. Eur J Neurosci. 2006;23:1839–1852. doi: 10.1111/j.1460-9568.2006.04710.x. [DOI] [PubMed] [Google Scholar]
- 69.Bukalo O, Fentrop N, Lee AY, Salmen B, Law JW, Wotjak CT, et al. Conditional ablation of the neural cell adhesion molecule reduces precision of spatial learning, long-term potentiation, and depression in the CA1 subfield of mouse hippocampus. J Neurosci. 2004;24:1565–1577. doi: 10.1523/JNEUROSCI.3298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood GK, Tomasiewicz H, Rutishauser U, Magnuson T, Quirion R, Rochford J, et al. NCAM-180 knockout mice display increased lateral ventricle size and reduced prepulse inhibition of startle. Neuroreport. 1998;9:461–466. doi: 10.1097/00001756-199802160-00019. [DOI] [PubMed] [Google Scholar]
- 71.Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, et al. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J Neurosci. 2010;30:4171–4183. doi: 10.1523/JNEUROSCI.5806-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demyanenko GP, Schachner M, Anton E, Schmid R, Feng G, Sanes J, et al. Close homolog of L1 modulates area-specific neuronal positioning and dendrite orientation in the cerebral cortex. Neuron. 2004;44:423–437. doi: 10.1016/j.neuron.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 73.Irintchev A, Koch M, Needham LK, Maness P, Schachner M. Impairment of sensorimotor gating in mice deficient in the cell adhesion molecule L1 or its close homologue, CHL1. Brain Res. 2004;1029:131–134. doi: 10.1016/j.brainres.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 74.Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 75.Ishiguro H, Saito T, Shibuya H, Toru M, Arinami T. Mutation and association analysis of the Fyn kinase gene with alcoholism and schizophrenia. Am J Med Genet. 2000;96:716–720. [PubMed] [Google Scholar]
- 76.Rybakowski JK, Borkowska A, Skibinska M, Hauser J. Polymorphisms of the Fyn kinase gene and a performance on the Wisconsin Card Sorting Test in schizophrenia. Psychiatr Genet. 2007;17:201–204. doi: 10.1097/YPG.0b013e3280991219. [DOI] [PubMed] [Google Scholar]
- 77.Hattori K, Fukuzako H, Hashiguchi T, Hamada S, Murata Y, Isosaka T, et al. Decreased expression of Fyn protein and disbalanced alternative splicing patterns in platelets from patients with schizophrenia. Psychiatry Res. 2009;168:119–128. doi: 10.1016/j.psychres.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 78.Yang LT, Alexandropoulos K, Sap J. c-SRC mediates neurite outgrowth through recruitment of Crk to the scaffolding protein Sin/Efs without altering the kinetics of ERK activation. J Biol Chem. 2002;277:17406–17414. doi: 10.1074/jbc.M111902200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.