Abstract
Transfusion medicine for the resuscitation of patients with massive hemorrhage has recently advanced from reactive, supportive treatment with crystalloid and red blood cell therapy to use of standardized massive transfusion protocols (MTPs). Through MTPs, medical facilities are able to standardize the most effective posthemorrhage treatments and execute them rapidly while reducing potential waste of blood products. Damage control resuscitation is an example of an MTP, where patients are (1) allowed more permissive hypotension, (2) spared large volumes of crystalloid/colloid therapy (through low volume resuscitation), and (3) transfused with blood products preemptively using a balanced ratio of plasma and platelets to red blood cells. This focused approach improves the timely availability of blood components during resuscitation. However, the use of MTPs remains controversial. This review describes published experiences with MTPs and illustrates the potential value of several MTPs currently utilized by academic transfusion services.
Massive hemorrhage is a common complication in a number of clinical settings. In traumatic injury, hemorrhage is a major cause of morbidity and is responsible for almost 50% of deaths occurring within 24 hours of injury and up to 80% of intraoperative trauma mortalities.1–3 In addition, cardiovascular and hepatobillary procedures can frequently result in massive bleeding4,5; postpartum hemorrhage events can complicate labor and delivery; and diverticulosis or varices can lead to significant gastrointestinal bleeding.5–8 Blood component support before and after control of massive hemorrhage is critical in these scenarios.7 Despite the need for consensus on the management of patients with massive bleeding, currently, no such consensus exists.
Traditionally, resuscitation has been initiated with large volumes of crystalloid, accompanied by packed red blood cell (pRBC) therapy.9 Plasma, platelets (PLTs), and cryoprecipitate are then supplemented based on laboratory values and at the discretion of the anesthesia and surgical teams. The goal of this treatment strategy is to treat coagulopathy after the patient is stabilized and the acute resuscitation is complete. However, studies suggest that even in cases where clinically apparent coagulopathy due to hemorrhage is addressed with early administration of plasma, the amount infused often falls significantly below what is needed to address the complex coagulopathy related to dilution, consumption, and fibrinolysis.10,11 Moreover, the use of large volume crystalloid support is independently associated with an increased volume of hemorrhage and lower survival rates.12–14
An estimated 10% of military trauma patients and 3% to 5% of civilian trauma patients receive massive transfusions (MTs), which is generally defined as more than 10 U of red blood cells (RBCs) within 24 hours of the start of treatment.6,15,16 This focus on the replenishment of RBCs does not address a significant subset of patients who would likely benefit from additional blood component therapy (ie, over a shorter interval).17 Both Moore et al17 and Holcomb et al6 demonstrated that patients receiving 10 U of pRBCs in the first 6 hours after injury had a higher rate of mortality than those receiving the same quantity of pRBCs over a 24-hour period. Early identification of this patient population and different MT strategies have been associated with improved survival.11
In response, transfusion services have implemented blood ordering protocols to quickly and efficiently provide sufficient amounts and types of blood products to patients with massive hemorrhage. There are a number of criteria available to help evaluate the effectiveness of different strategies used by transfusion services. Evaluation of the effectiveness of these protocols should include several parameters: clinical outcomes (survival, length of hospital stay, multisystem organ failure, infection rate, etc); postresuscitation laboratory parameters [hemoglobin, prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, and PLT count]; and 24-hour blood component and crystalloid use. Our review summarizes the literature to date, highlights some of the limitations of the available evidence, and identifies areas in need of additional study.
EARLY INTERVENTION HELPS ALLEVIATE COAGULATION DISTURBANCES
Military and civilian studies show that the presence of coagulopathy is associated with poorer outcomes in patients with severe hemorrhage.18 Up to 25% of trauma patients exhibit abnormal coagulation parameters at the time of presentation, which is associated with a 3-fold increase in mortality.19,20 Military patients who had an international normalized ratio of more than 1.5 had a mortality of 30%, compared with 5% in those with hemorrhage but a normal international normalized ratio at presentation.18,21 There are multiple factors that contribute to disturbances in coagulation. Immediately after injury, hypoperfusion influences coagulopathy through tissue injury/reperfusion, consumption, and increased fibrinolysis.9,22,23 Hemodilution also occurs and is associated with crystalloid and pRBC treatment administered without concomitant plasma component therapy.9 Plasma therapy early in resuscitation could lessen the development of coagulopathy via reduction of the severity of blood loss and amelioration of ongoing bleeding.
Little is known about coagulopathy in nontrauma patients with massive exsanguination. The underlying condition that induces exsanguination (eg, liver dysfunction, obstetric cases) or related comorbidities (eg, uremia, use of anticoagulants or anti-PLT agents) significantly impacts the patient’s coagulation status and need for blood components. Coagulopathy can be exacerbated if resuscitation is initiated and continued with only crystalloid and RBC therapy, whereas early, proactive resuscitation with plasma and PLTs may improve outcomes.
There is literature that the rapid treatment of initial coagulation disturbances improves survival.19,24 In the trauma literature, clinical studies emphasize the importance of identification and treatment of coagulopathy in the early stages of presentation.18 On the other hand, some studies of patients treated early with plasma and PLTs show variable outcomes and with recommendations that plasma transfusions be withheld until the PT or activated PTT time is 1.5 times normal.22,25 However, laboratory-guided component therapy is limited as a decision-guiding tool during cases of rapid, massive exsanguination.26 There can be a significant interval between the time that tests are ordered and results are available. In addition, after a decision is made by the clinical team to provide plasma, additional time (approximately 30–45 minutes) may be required to thaw and issue multiple units of blood type-specific plasma. In the severely bleeding or injured patient, there may be a missed opportunity to prevent or manage coagulopathy. Thus, laboratory-guided component therapy is limited as a decision-guiding tool during cases of rapid, massive exsanguination.26
Although many institutions have embraced the concept of “proactive” resuscitation and earlier use of blood and blood components, transfusion of these products may be associated with morbidity and mortality. Several reports have noted that these blood components may be associated with an increased risk of infection and organ failure.23,27–31 Recently, Sarani et al29 evaluated nontrauma surgical intensive care patients and noted that transfusion of plasma was associated with an increased risk of infectious complications, with a 4% increase in the odds risk of infection per unit of plasma. Similarly, another report found the risk of pneumonia to be increased by 5% for each unit of blood or PLTs transfused.32 However, when these products are transfused early as part of a protocol for trauma patients, the overall number of blood products transfused in the first 24 h actually decreased.33,34 With fewer units of exposure, use of a protocol for patients with massive hemorrhage was associated with a reduction in risk of organ failure and postinjury complications in one study.35
Motivation to standardize resuscitation has generated the development of massive transfusion protocols (MTPs) in which the surgical or medical team facilitates the transfusion of pRBC, plasma, and PLTs in predetermined and standardized ratios. Protocols and ratios of blood components and their administration may differ among medical facilities but are intended to continue treatment until hemorrhage is controlled.6,33,36 Studies are needed to compare protocols to improve patient outcomes. To illustrate the limitations and advantages of specific MTPs, we describe our experiences with MTPs at our own institutions.
TWO INSTITUTIONAL PERSPECTIVES ON MTPs
Institution 1
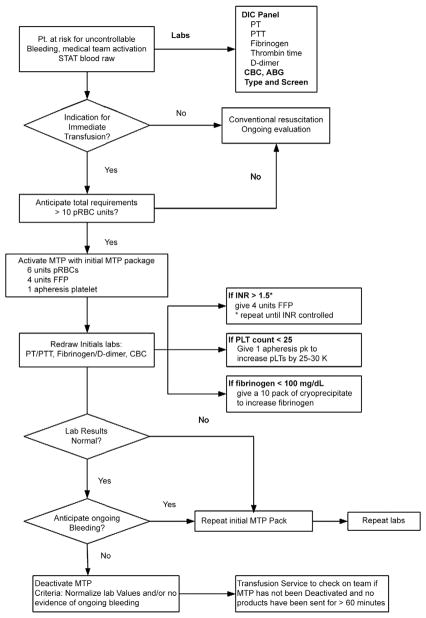
An MTP was established initially for trauma and labor and delivery and later for surgical and critical care patients, which provides for emergency release of 6 U of pRBCs, 4 U of plasma (liquid plasma, p24 plasma, or 5 day plasma), and 1 apheresis platelet (aPLT) unit (Fig 1).7 This order set is compiled and electronically issued within 6–10 minutes after the verbal telephone order to activate the protocol. To facilitate execution of the MTP, 4 U of blood type A and O thawed plasma are also available. If AB or B plasma is needed (or patient blood type is unknown), liquid AB plasma (with a shelf life of 28 days) is provided. The 6:4:1 ratio of pRBCs, plasma, and aPLT (functionally a 6:5:1 ratio since an aPLT contains approximately 1 U of plasma) was designed to achieve replacement of 70% of RBC volume and 60% of circulating plasma volume for a 70-kg individual.
Fig 1.
Institution 1 MTP algorithm. The clinical team sends blood samples for initial laboratory tests to the clinical laboratory upon initiation of the MTP. The tests requested include PT, PTT, fibrinogen, D-dimer, and a CBC. If a patient continues to hemorrhage or future hemorrhage is anticipated, additional 6:4:1 packages can be requested from the transfusion service, provided that additional coagulation laboratory kits are sent with each request. Importantly, the massive transfusion guideline does not preclude customized ordering of other blood products and pharmaceuticals. Later in resuscitation, abnormal laboratory values such as prolonged PT and/or PTT, low PLT count, and low fibrinogen values can be addressed individually as depicted. Figure revised with permission from Burtelow et al.7
Although blood for diagnostic laboratory evaluation is recommended as part of this protocol, the treatment of patients with blood components does not depend on laboratory test results. Note in Figure 1 that initial laboratory tests are prescribed at initiation of the MTP and include PT, PTT, fibrinogen, D-dimer, and a complete blood count (CBC). If a patient continues to hemorrhage and/or additional MTP packages are requested, additional laboratory specimens are ordered. The MTP does not preclude customized ordering of other blood products (eg, cryoprecipitate) and/or pharmaceuticals (eg, recombinant factor 7a, rVIIa). Because of the time interval from blood draw to assay results, the intent of these coagulation assays is to provide support for the value of the MTP as intended or evidence that modification of the MTP may be needed.
Institution 2
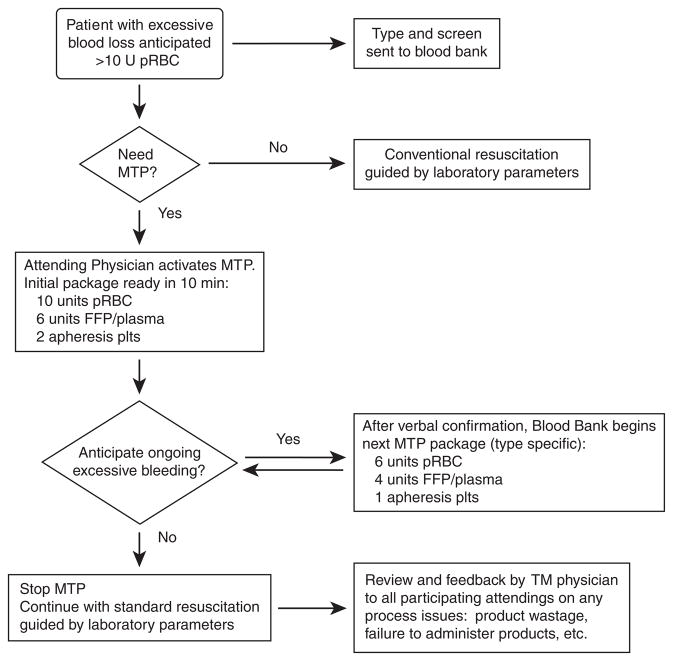
An MTP instituted in 2005 for the trauma service has subsequently been made available to all surgical and obstetric services at one of our medical centers (Fig 2).11,33,37 The protocol is activated by a verbal telephone order from an attending physician to the blood bank. The blood bank prepares 6 U of group O pRBCs, 4 U of thawed plasma, and 1 U of aPLT (effectively a ratio of 6:5:1 of pRBC/plasma/aPLT) and delivers them within 10 minutes of the call. To provide expedient service for trauma patients with unknown blood types, 4 U of thawed AB plasma are inventoried. A specimen for blood type and screen is sent at initiation of resuscitation. For nontrauma patients, the blood type and screen results are often available before MTP initiation. Administration of type-specific blood products helps avoid excessive utilization of Group O pRBCs and AB plasma, which is an important determinant for quality management programs.11 If continued resuscitation is needed, then a ratio of 6:4:1 of pRBC/plasma/aPLT are delivered in 30-minute intervals until the protocol is discontinued. Typically, 3 cycles of blood products are prepared and only 2 are actually issued or administered to the patient. Additional ordering of other blood products is ideally suspended during the MTP, which conserves resources during the early period of the MTP and helps to prevent delay in the preparation of the next cycle of components.11 Once hemorrhage is controlled, the MTP is discontinued, and further transfusions should be guided by clinical and laboratory assessment. Each MTP activation and execution is reviewed by a transfusion medicine service physician within 24 hours, in which feedback is initiated with participants involved in the MTP. The hospital transfusion committee also provides oversight related to the MTP, including product wastage and noncompliance to infuse components in designated ratios.11
Fig 2.
Institution 2 MTP algorithm. This figure illustrates the MTP used at a second institution. This protocol can be activated by any trauma surgeon, but more recently, its use has been extended to other surgical services. TM indicates transfusion medicine.
We surveyed members of the University Health System Consortium, an alliance of 107 academic medical centers with 232 affiliated hospitals, to evaluate the use of MTP protocols at other institutions. There were 25 respondents, and 14 representative questionnaire responses are summarized in Table 1. The majority (70%) used a 1:1 ratio of pRBC/plasma for the initial cycle, although for some, the ratios changed from cycle to cycle. The routine use of cryoprecipitate and rVIIa occurred in 36% and 15 % of the protocols, respectively; for most, these were issued upon request. Importantly, thawed plasma sufficient to provide at least a portion of the plasma required for the first cycle was available in only 60% of the responding hospitals, indicating significant delay in the use of plasma during the early phases of resuscitation. All respondents attempted to switch to blood type–specific pRBC when possible. However, it was noted that for institutions requiring an independent sample for ABO verification, the change to type-specific products may not occur during a trauma resuscitation because these patients are usually not previously known to the hospital and a historic blood type is unavailable. Although the number of respondents was small and the survey does not provide insight into the prevalence of MTPs at academic hospitals, the results do provide a current snapshot and reflect significant variation in practice. This variation indicates that consensus practice guidelines are needed.38,39
Table 1.
Massive Transfusion Protocols in Use at Various Academic Medical Centers
| Hospital | pRBC/FFP/PLT | Cryoprecipitate use? | rFVIIa use? | Thawed plasma immediately available? | Conversion to type-specific products when possible? |
|---|---|---|---|---|---|
| 1 | Cycle 1: 6:6:6 (random) Cycle 2: 6:6:0 Cycle 3: 6:6:0 Cycle 4: 6:6:6 (random) Cycle 5: 6:6:0 Cycle 6: 6:6:0 |
Cycle 2: 20 U Cycle 5: 10 U |
Cycle 3: 40 μg/kg Cycle 6: 40 μg/kg |
No | Yes |
| 2 | 5:2:1 (apheresis) | Every third cycle: 5 U | Every other cycle: 2 mg | Yes | Yes |
| 3 | 6:4:2 | Upon request | No | No | Yes |
| 4 | 4:4:0 | No | No | No | Yes |
| 5 | Cycle 1: 6:6:1 (apheresis) Cycle 2: 6:6:1 Cycle 3: 6:6:0 Cycle 4: 6:6:1 Cycle 5: 6:6:0 Cycle 6: 6:6:1 |
Cycle 1: 10 U Cycle 3: 10 U Cycle 5: 10 U |
No | No | Yes |
| 6 | 5:5:1 (apheresis) | No | No | Yes | Yes |
| 7 | Cycle 1: 5:3:0 Cycle 2: 5:3:0 Cycle 3: 5:3:1 (apheresis) Cycle 4: 5:3:0 Cycle 5: 5:3:1 Cycle 6: 5:3:0 |
No | No | Yes | Yes |
| 8 | 12:12:1 (apheresis) | Upon request | No | Yes | Yes |
| 9 | 4:4:4 (random) | Upon request | No | No | Yes |
| 10 | 6:6:1 (apheresis) | Every third cycle: 10 U | No | Yes | Yes |
| 11 | Cycle 1: 4:4:0 Subsequent cycles: 4:4:1 |
No | No | Yes | Yes |
| 12 | Cycle 1: 5:5:0 Cycle 2: 5:5:1 Cycle 3: 5:5:0 Cycle 4: 5:5:1 Cycle 5: 5:5:0 Cycle 6: 5:5:1 |
Every 3rd cycle: 10 U | No | Yes | Yes |
| 13 | Cycle 1: 10:6:2 (apheresis) Subsequent cycles: 6:4:1 |
Upon request | No | Yes | Yes |
| 14 | Cycle 1: 6:6:1 (apheresis) Cycle 2: 6:6:0 Cycle 3: 6:6:1 Cycle 4: 6:6:0 |
Upon request | Upon request 90 μg/kg |
Yes | Yes |
IMPACT ON PATIENT OUTCOMES
Recent data from the US Army’s Institute of Surgical Research have shown improvement in outcomes when soldiers requiring MTs received resuscitations with ratios of component types that were similar to whole-blood transfusions.40,41 Subsequent reports, primarily in the military literature, further supported a component therapy transfusion in ratios of 1 U pRBC/1 U plasma/1 random donor unit of PLTs.6,24,42,43 Casualties who received less than 1 U of plasma for every 4 U of pRBC were associated with a 65% mortality, whereas those who received 2 U of plasma for every 3 U of pRBCs were associated with 19% mortality.9 The military data have the strength of large numbers of casualties requiring MT. However, these are retrospective studies, and soldiers who died before receiving sufficient (intended) thawed plasma were grouped in the low ratio group, hence introducing a survivorship bias.6,24,42,43 The military data also lacked adjustment for confounding variables with mortality, such as injury severity.6,24,42,43 It is interesting to note that, in one of the military studies, although the goal was a ratio of 1:1, the patients more commonly reached a ratio of 3:2, which is also the ratio supported as an optimum minimum ratio by computer models to avoid dilutional hemorrhage.15,44 Nevertheless, these data have led to widespread support of this ratio, particularly the 1:1 ratio of pRBC/plasma, although considerable debate remains on this topic.26,39,45
Civilian trauma studies (a portion of which are summarized in Table 2) have also evaluated the impact of more aggressive ratios and noted an association with improved survival with use of MTPs.22,33,46,47 Similar to military data, such studies also support using a more aggressive pRBC/plasma ratio, albeit the precise ratio varied significantly among groups.48 The use of a pRBC/plasma ratio between 3:2 and 1:1 (n = 196) resulted in a significant reduction (41% vs 62%) in 30 day mortality as compared with those that received less plasma.37 In that study, an increased odds of survival was associated with an increased intraoperative pRBC/plasma ratio between 3:2 and 1:1.37 This was independent of age and Trauma Related Injury Severity Score, which by themselves were independent predictors of mortality as well.37 The same study favored a PLT ratio of 1 pRBC: 1 random donor pool (or 5 pRBCs: 1 aPLT).37 Interestingly, while the intraoperative product use was higher than the non-MTP group, the 24-hour overall blood component usage was significantly lower as a result of statistically significantly lower utilization of PLTs in the MTP arm (6.8 vs 3.1, P < .001). The postoperative PTT and PLT counts, available only on a subset of study patients, were also significantly better in the MTP versus non-MTP cohort (39 vs 33 seconds, P = .38; PLT count 136 vs 96, P = .001; B.A.C., unpublished data). The intraoperative crystalloid use was also significantly lower in the MTP arm (6.7 vs 4.9 L, P = .002).33 There is also some anecdotal evidence that MTPs may more effectively manage acidemia and normalize lactate levels in trauma patients (B.A.C. and P.P.Y., unpublished observations).
Table 2.
Studies Evaluating the Impact of an MTP on Outcomes in Trauma Patients
| Author(s) | Design | Number of MT Patients | Ratios used (PRBC/Plasma/PLT) | Impact of MTP on Outcomes | Limitations and Comments |
|---|---|---|---|---|---|
| Cotton et al (2008) | Retrospective (before and after) cohort study, single civilian center | 264 | 3:2:3 | Reduction in 24-hour blood utilization, 30-day mortality, multiple-organ failure, and abdominal compartment syndrome | Single center, retrospective, no standardized activation criteria |
| Cotton et al (2009) | Prospective cohort study, single civilian center | 125 | 3:2:3 | Poor compliance with MTP guidelines is associated with worse outcomes; failure to activate MTP early and to transfuse predefined ratios of plasma and PLTs associated with higher 24-hour and 30-day mortality | Single center, no standardized activation criteria. Prospective examination of protocol compliance and provider-related factors and their impact on patient outcomes |
| Dente et al (2009) | Retrospective (before and after) cohort study, single civilian center | 157 | 1:1:1 | Reduction in 24-hour mortality and reduction in 30-day mortality in blunt trauma | Single center, retrospective with historical controls, high MTP failure rate |
| Duchesne et al (2009) | Retrospective registry review, single civilian center | 435 | 1:1:1 | Reduction in 24-hour and 30-day mortality | Single center, retrospective but has standardized activation criteria |
| O’ Keeffe et al (2008) | Retrospective (before and after) cohort study, single civilian center | 132 | 5:2:3 | Reduction in blood and blood component utilization, time to delivery of initial products, and hospital charges | Single center, retrospective, no standardized activation criteria; also used cryoprecipitate and rFVIIa |
| Riskin et al (2009) | Retrospective (before and after) cohort study, single civilian center | 77 | 3:2:3 | Reduction in time to delivery of initial products and 30-day mortality | Small study size, single center, retrospective, no standard activation criteria |
A similar 3:2 pRBC/plasma ratio was used in an MTP protocol for postpartum hemorrhage in obstetric patients.7 Dente and colleagues47,49 evaluated an MTP for civilian trauma patients designed to achieve a pRBC/plasma ratio of 1:1. Although a ratio of 1:1 was their goal, their MTP patients actually received a 3:2 pRBC/plasma ratio, whereas the non-MTP arm received a 3:1 pRBC/plasma ratio. Among blunt trauma patients, the MTP group (with higher plasma portions) exhibited significantly lower 24-hour and 30-day mortality than a control cohort with similar injury severity scores and less plasma. Early crystalloid use was also significantly less in the MTP group.49 Johansson et al50 demonstrated that patients with severe bleeding secondary to ruptured aortic aneurysms showed improved survival when treated with 1:1 ratio of pRBC to plasma.
However, several studies have called into question the benefit of higher ratios.46,51 Shortly after military and civilian data advocated for earlier use and higher ratios of plasma products in MT patients, Kashuk et al46 argued against such ratios. In their study, patients receiving pRBC to plasma in a ratio between 2:1 and 1:1 had a lower 30-day mortality, whereas those with 1:1 had higher mortality. The authors proposed that while patients may benefit from more plasma, aiming for 1:1 was not beneficial and was, in fact, associated with higher mortality. Notably, their study included 133 patients, of which only 11 patients achieved a 1:1 ratio. More recently, Snyder et al51 suggested that the results of studies evaluating the impact of damage control resuscitation and their associated higher ratios of products may have found benefit solely through “survival bias.” However, many of the recent studies examining MTPs and higher ratios actually exclude patients that (1) do not survive the initial operation and resuscitation (approximately the first 3 hours) and (2) do not receive at least 10 U of products before death, specifically to reduce the role of “survival bias” impact on outcome.33,37,39,52
Beyond the impact on survival, there are data to suggest that MTPs shorten both the time for delivery of first shipment of products as well as delivery of subsequent shipments to the bedside.25,34 As mentioned above, the aggressive, early administration of a predesigned complement of blood products also appears to decrease overall blood use and therefore, blood component costs,33,34 if not survival.53 Studies are needed to evaluate these aspects of MTP use.
Although a previously published poll of trauma centers found established protocols in a relatively small number of well-organized centers,54 it is likely that an increasing number of transfusion services, especially those with trauma centers, have a defined MTP. Although such protocols have been associated with improved survival, it is important to assess the data carefully because many centers with MTPs have a history of poor compliance during active resuscitations.11,15,37,49,54 As noted previously, Borgman et al15 noted that while the intended ratio for delivered products was 1:1:1 (pRBC/plasma/random donor PLT unit), fewer than 20% of patients actually received these ratios. Similarly, only one third of patients in a recent civilian study received the predefined ratios of plasma, and fewer than a quarter received the specified PLT ratios.11 More concerning was the finding that failure to achieve these ratios was associated with higher mortality in both military and civilian settings.11,15,37 Thus, when evaluating MTPs, both the protocol and the compliance should be considered before making any conclusions about patient outcomes. Moreover, the timing of activation (ie, whether the protocol is proactively activated early in resuscitation versus late) will likely impact the type of components needed and may have an impact on patient outcomes as well. These findings suggest that multidisciplinary oversight and training should include representatives from transfusion medicine, surgery (in particular, the trauma service), anesthesiology, and critical care to ensure that a well-designed MTP is actually utilized as intended. Additional guidance in the literature on how to develop such oversight strategies would be valuable.
In summary, the cumulative data support early, proactive support with high ratios of plasma to pRBC along with additional support with PLTs.26,39 Not all studies showed a mortality benefit, and in the absence of randomized trials, data to convincingly support a particular ratio or formula are needed. However, the existing data suggest that a well-organized MTP protocol that is activated in a timely fashion is likely to demonstrate improved patient outcomes and result in less overall blood product usage in large trauma centers.
ADJUNCT STRATEGIES
Fresh whole blood has been successfully utilized in austere environments where component therapy is not available or has been depleted.1,55,56 In the most recent conflict in Iraq, military investigators evaluated 100 patients who received whole-blood resuscitation and compared them with 254 patients who received component therapy. The authors found that fresh whole blood performed well compared with component-based resuscitation in patients who required MT, reporting improved 24-hour and 30-day survival.57 Some military researchers advocate its use for these scenarios and have found it to be “convenient, safe, and effective.”1 In fact, some investigators propose that fresh whole blood is likely to be superior, as it is likely to provide fresher units than standard components.56 However, while used successfully in some military resuscitation settings (with readily available, prescreened donor pool), most US trauma centers and their blood product suppliers do not currently consider this a feasible alternative because of its inability to perform pretransfusion testing of donor units, risk of hemolysis from out-of-group plasma, and few readily available donors. Furthermore, the absence of prospective clinical trials in demonstrating superiority to component therapy argues against its use.
There are little published data that specifically address the utility of other components or special factors such as recombinant human factor VIIa (rFVIIa) in an MTP.58–60 A large manufacturer-sponsored trial involving 150 international sites that was designed to assess the potential for mortality and morbidity benefits associated with use of rFVIIa in blunt and penetrating trauma was recently terminated when a preplanned interim data analysis predicted a low likelihood of observing a significant difference in the primary mortality end point.61 Dosing in this trial was 200 μg once and followed by 100 μg at 1 and 3 hours following the initial dose. Before termination of the trial, 218 patients were treated with rFVIIa and 242 received placebo for blunt trauma and the mortality rate was 11% in both groups (P = .934). Too few patients with penetrating trauma were enrolled to permit statistical analyses.
Cryoprecipitate is enriched in von Willebrand factor/VIII complex, factor XIII, and most importantly, fibrinogen. Cryoprecipitate is administered as a supplement to plasma to increase plasma fibrinogen. Hypofibrinogenemia is not likely to contribute to bleeding until the level falls below 100 mg/dL; hence, cryoprecipitate is recommended for patients with documented decrease in fibrinogen below 100 mg/dL. It is estimated that a dose of 8–10 U of cryoprecipitate will increase the fibrinogen in a 70 kg adult by 50–70 mg/dL. Each unit of fresh frozen plasma (FFP)/plasma contains 0.5 g of fibrinogen as well as all other coagulation proteins. Hence, provided the resuscitation with plasma (in the form of MTP, for instance) is initiated early, continued administration of high ratios of plasma should be able to ensure hemostatic concentration of any component present in cryoprecipitate, including fibrinogen. In the setting of ongoing and massive hemorrhage, volume constraints for plasma therapy are usually not a consideration. This further highlights the importance of considering the timing of activation (proactive vs reactive) in the design of the optimal MTP.
In the recently published CRASH-2 study, the antifibrinolytic agent tranexamic acid was shown to reduce mortality when administered to trauma patients.62 Whether this effect was due to improved hemostasis (although no differences in blood transfusions were observed) or to other anti-inflammatory effects is unknown. This trial provides insight into the difficulty for a clinical trial in the trauma setting to be able to demonstrate marginal differences in mortality (1.4 for every 100 patients treated) or any other potential clinical benefit with any particular intervention. Related and additional challenges include the difficulties with informed consent, adherence to clinical trial designs that include over or under accrual of patients (triage failures), product wastage, and complex treatment algorithms.
CHALLENGES ASSOCIATED WITH IMPLEMENTATION OF MTPs AND THE FOCUS OF FUTURE RESEARCH
Adequately powered studies in which the analysis adjusts for injury severity and survivorship bias are necessary in both military and civilian settings to clearly define the optimal ratio of pRBC/plasma in the trauma population. Such studies should also be designed to examine the utility of other adjunct agents such as cryoprecipitate and rFVIIa. Moreover, thus far, virtually all of the literature has focused on trauma hemorrhage. However, there are likely to be differences in the manner in which MTPs are used in trauma versus cardiovascular, gastrointestinal, and other surgical settings. One distinction between trauma services with other resuscitative efforts is that trauma surgeons will often take a proactive strategy at presentation. Massive transfusion protocols for other cases may be more often activated reactively after a prolonged period of ongoing hemorrhage and resuscitation with crystalloid and pRBCs.
Evaluation of effectiveness of MTP protocols to address massive hemorrhage should include several parameters: clinical outcomes (survival, length of hospital stay, multisystem organ failure, infection rate, etc.) in conjunction with post resuscitation laboratory parameters (hemoglobin, PT/PTT, fibrinogen, and PLT count) as well as 24-hour/total blood component and crystalloid use. Future trials are also needed to understand the basis of enhanced survival. In theory, MTPs prevent coagulopathy, and they also appear to decrease overall blood component use and, importantly, crystalloid use, which has been independently linked with poor outcome.33 Elucidating the potential mechanism behind improved survival with use of MTP protocols delivering higher ratios of plasma to pRBC will ultimately improve our overall understanding of pathophysiology of massive hemorrhage.
CONCLUSIONS
Current trauma resuscitation of the severely injured patient focuses on restoration of clotting factors, as well as depleted and dysfunctional PLTs through the concept of damage control resuscitation, with proactive transfusion of higher ratio of plasma and PLTs to red blood cells.37 Massive transfusion protocols with higher ratios of plasma and PLTs to pRBCs appear to be associated with improved survival in patients with massive hemorrhage. Further research into this important topic is needed, both on a scientific level to determine the mechanism underlying improvement in survival, as well as in the systems management level, to ensure compliance with these protocols.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations and therapeutic considerations. J Trauma. 2006;60(6 suppl):S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Acosta JA, Yang JC, Winchell RJ, et al. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 3.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: A reassessment. J Trauma. 1995:185–193. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Trowbridge C, Stammers A, Klayman M, et al. Characteristics of uncontrolled hemorrhage in cardiac surgery. J Extra Corpor Technol. 2008;40:89–93. [PMC free article] [PubMed] [Google Scholar]
- 5.Rose AH, Kotze A, Doolan D, et al. Massive transfusion-evaluation of current clinical practice and outcome in two large teaching hospital trusts in Northern England. Vox Sang. 2009;97:247–253. doi: 10.1111/j.1423-0410.2009.01198.x. [DOI] [PubMed] [Google Scholar]
- 6.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: Directly addressing the early coagulopathy of trauma. J Trauma. 2007;62:307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 7.Burtelow M, Riley E, Druzin M, et al. How we treat: Management of life-threatening primary postpartum hemorrhage with a standardized massive transfusion protocol. Transfusion. 2007;47:1564–1572. doi: 10.1111/j.1537-2995.2007.01404.x. [DOI] [PubMed] [Google Scholar]
- 8.Barnert J, Messmann H. Medscape: Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2009;6:637–646. doi: 10.1038/nrgastro.2009.167. [DOI] [PubMed] [Google Scholar]
- 9.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23:231–240. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geeraedts LM, Demiral H, Schaap NP, et al. “Blind” transfusion of blood products in exsanguinating trauma patients. Resuscitation. 2007;73:382–388. doi: 10.1016/j.resuscitation.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Cotton BA, Dossett LA, Au BK, et al. Room for performance improvement: Provider-related factors associated with poor outcomes in massive transfusion. J Trauma. 2009;67:1004–1012. doi: 10.1097/TA.0b013e3181bcb2a8. [DOI] [PubMed] [Google Scholar]
- 12.Kowalenko T, Stern S, Dronen S, et al. Improved outcome with hypotensive resuscitation of uncontrolled hemorrhagic shock in a swine model. J Trauma. 1992;33:349–353. [PubMed] [Google Scholar]
- 13.Balogh Z, McKinley BA, Holcomb JB, et al. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J Trauma. 2003;54:848–859. doi: 10.1097/01.TA.0000070166.29649.F3. [DOI] [PubMed] [Google Scholar]
- 14.Joshi GP. Intraoperative fluid restriction improves outcome after major elective gastrointestinal surgery. Anaesth Analg. 2005;101:601–605. doi: 10.1213/01.ANE.0000159171.26521.31. [DOI] [PubMed] [Google Scholar]
- 15.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused effects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 16.Como JJ, Dutton RP, Scalea TM, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004;44:809–813. doi: 10.1111/j.1537-2995.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 17.Moore FA, Nelson T, McKinley BA, et al. Massive transfusion in trauma patients: Tissue hemoglobin oxygen saturation predicts poor outcome. J Trauma. 2008;64:1010–1023. doi: 10.1097/TA.0b013e31816a2417. [DOI] [PubMed] [Google Scholar]
- 18.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64:1459–1463. doi: 10.1097/TA.0b013e318174e8bc. [DOI] [PubMed] [Google Scholar]
- 19.Brohi K, Singh J, Heron M, et al. Acute traumatic coagulopathy. J Trauma. 2003;54:1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 20.Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: An analysis of the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Stinger HK, Spinella PC, Perkins JG, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;24(suppl):S79–S85. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- 22.Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137:209–220. doi: 10.1378/chest.09-0252. [DOI] [PubMed] [Google Scholar]
- 23.Norda R, Tynell E, Akerblom O. Cumulative risks of early fresh frozen plasma, cryoprecipitate and platelet transfusion in Europe. J Trauma. 2006;60(6 suppl):S41–S45. doi: 10.1097/01.ta.0000199546.22925.31. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber MA, Perkins JG, Kiraly L, et al. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007;64:541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Riskin DJ, Tsai TC, Riskin L, et al. Massive transfusion protocols: The role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209:198–203. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 26.Zielinski M, Park MS, Jenkins D. Appropriate evidence-based practice guidelines for plasma transfusion would include a high ratio of plasma to red blood cells based on the available data. Transfusion. 2010;50:2762–2764. doi: 10.1111/j.1537-2995.2010.02806.x. [DOI] [PubMed] [Google Scholar]
- 27.Dunne JR, Riddle MS, Danko JH, et al. Blood transfusion is associated with infection and increased resource utilization in combat casualties. Am Surg. 2006;72:619–625. [PubMed] [Google Scholar]
- 28.Vamvakas EC. Platelet transfusion and postoperative infection in cardiac surgery. Transfusion. 2007;47:352–354. doi: 10.1111/j.1537-2995.2007.01115.x. (letter); author reply 354–356. [DOI] [PubMed] [Google Scholar]
- 29.Sarani B, Dunkman WJ, Dean L, et al. Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit Care Med. 2008;36:1114–1118. doi: 10.1097/CCM.0b013e318168f89d. [DOI] [PubMed] [Google Scholar]
- 30.Moore FA, Sauaia A, Moore EE, et al. Postinjury multiple organ failure: A bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132:620–624. [PubMed] [Google Scholar]
- 32.Vamvakas EC, Carven JH. Transfusion and postoperative pneumonia in coronary artery bypass graft surgery: Effect of the length of storage of transfused red cells. Transfusion. 1999;39:701–710. doi: 10.1046/j.1537-2995.1999.39070701.x. [DOI] [PubMed] [Google Scholar]
- 33.Cotton BA, Gunter OL, Isbell J, et al. Damage control hematology: The impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–1183. doi: 10.1097/TA.0b013e31816c5c80. [DOI] [PubMed] [Google Scholar]
- 34.O’Keefe T, Refaai M, Tchorz K, et al. A massive transfusion protocol to decrease blood component use and costs. Arch Surg. 2008;143:686–690. doi: 10.1001/archsurg.143.7.686. [DOI] [PubMed] [Google Scholar]
- 35.Cotton BA, Au BK, Nunez TC, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma. 2009;66:41–48. doi: 10.1097/TA.0b013e31819313bb. [DOI] [PubMed] [Google Scholar]
- 36.Malone DL, Hess JR, Fingerhut A. Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J Trauma. 2006;60(6 suppl):S91–S96. doi: 10.1097/01.ta.0000199549.80731.e6. [DOI] [PubMed] [Google Scholar]
- 37.Gunter OL, Au BK, Isbell JM, et al. Optimizing outcomes in damage control resuscitation: Identifying blood product ratios associated with improved survival. J Trauma. 2007;65:527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 38.Schuster KM, Davis KA, Lui FY, et al. The status of massive tranfsuion protocols in the United States trauma centers: Massive transfusion or massive confusion. Transfusion. 2010;50:1545–1551. doi: 10.1111/j.1537-2995.2010.02587.x. [DOI] [PubMed] [Google Scholar]
- 39.Stansbury LG, Dutton RP, Stein DM, et al. Controversy in trauma resuscitation: Do ratios of plasma to red blood cells matter? Transfus Med Rev. 2009;23:255–265. doi: 10.1016/j.tmrv.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Hess JR, Zimrin AB. Massive blood transfusion for trauma. Curr Opin Hematol. 2005;12:488–492. doi: 10.1097/01.moh.0000177828.85904.70. [DOI] [PubMed] [Google Scholar]
- 41.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: The need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46:685–686. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 42.Dutton RP, Carson JL. Indications for early red blood cell transfusion. J Trauma. 2006;60(6 suppl):S35–S40. doi: 10.1097/01.ta.0000199974.45051.19. [DOI] [PubMed] [Google Scholar]
- 43.Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate and platelet transfusion in trauma. J Trauma. 2006;60(6 suppl):S1–S58. doi: 10.1097/01.ta.0000199432.88847.0c. [DOI] [PubMed] [Google Scholar]
- 44.Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: A computer simulation. J Trauma. 2003;54:454–463. doi: 10.1097/01.TA.0000053245.08642.1F. [DOI] [PubMed] [Google Scholar]
- 45.Callum JL, Nascimento B, Tien H, et al. Editorial: “Formula-driven” versus “lab-driven” massive transfusion protocols: At the state of clinical equipoise. Transfus Med Rev. 2009;23:247–254. doi: 10.1016/j.tmrv.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: Is 1:1 fresh frozen plasma: Packed red blood cells the answer? J Trauma. 2008;65:261–271. doi: 10.1097/TA.0b013e31817de3e1. [DOI] [PubMed] [Google Scholar]
- 47.Shaz BH, Dente CJ, Nicholas JM, et al. Increased number of coagulation products in relationship to red blood cell products transfused imporves mortality in trauma patients. Transfusion. 2010;50:493–500. doi: 10.1111/j.1537-2995.2009.02414.x. [DOI] [PubMed] [Google Scholar]
- 48.Sperry JL, Ochoa JB, Gunn SR, et al. FFP:PRBC transfusion ratio >1:1.5 is associated with lower risk of mortality after massive transfusion. J Trauma. 2008;65:986–993. doi: 10.1097/TA.0b013e3181878028. [DOI] [PubMed] [Google Scholar]
- 49.Dente CJ, Shaz BH, Nicholas JM, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66:1616–1624. doi: 10.1097/TA.0b013e3181a59ad5. [DOI] [PubMed] [Google Scholar]
- 50.Johansson PI, Stensballe J, Rosenberg I, et al. Proactive adminstration of platelets and plasma for patients with a ruptured abdominal aortic aneurysm: Evaluating a change in transfusion practice. Transfusion. 2007;47:593–598. doi: 10.1111/j.1537-2995.2007.01160.x. [DOI] [PubMed] [Google Scholar]
- 51.Snyder CW, Weinberg JA, McGwin G, et al. The relationship of blood product ratio to mortality: Survival benefit or survival bias? J Trauma. 2009;66:358–362. doi: 10.1097/TA.0b013e318196c3ac. [DOI] [PubMed] [Google Scholar]
- 52.Duchesne JC, Kimonis K, Marr AB, et al. Damage control resuscitation in combination with damage control laparotomy: A survival advantage. J Trauma. 2010;69:46–52. doi: 10.1097/TA.0b013e3181df91fa. [DOI] [PubMed] [Google Scholar]
- 53.Johansson PI. Hemostatic strategies for minimizing mortality in surgery with major blood loss. Curr Opin Hematol. 2009;16:509–514. doi: 10.1097/MOH.0b013e32833140fc. [DOI] [PubMed] [Google Scholar]
- 54.Hoyt DB, Dutton RP, Hauser CJ, et al. Management of coagulopathy in the patients with multiple injuries: Results from an international survey of clinical practice. J Trauma. 2008;65:755–764. doi: 10.1097/TA.0b013e318185fa9f. [DOI] [PubMed] [Google Scholar]
- 55.Spinella PC. Warm fresh whole blood transfusion for severe hemorrhage: US military and potential civilian applications. Crit Care Med. 2008;36(7 suppl):S340–S345. doi: 10.1097/CCM.0b013e31817e2ef9. [DOI] [PubMed] [Google Scholar]
- 56.Spinella PC, Perkins JG, Grathwohl KW, et al. Risks associated with fresh whole blood and red blood cell transfusions in a combat support hospital. Crit Care Med. 2007;35:2576–2581. doi: 10.1097/01.CCM.0000285996.65226.A9. [DOI] [PubMed] [Google Scholar]
- 57.Spinella PC, Perkins JG, Grathwohl KW, et al. Warm fresh blood is independently associated with improved survival for patients with combat-related traumatic injuries. J Trauma. 2009;66 (4 suppl):S69–S76. doi: 10.1097/TA.0b013e31819d85fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perkins JG, Cap AP, Weiss BM, et al. Massive transfusion and nonsurgical hemostatic agents. Crit Care Med. 2008;36(7 suppl):S325–S339. doi: 10.1097/CCM.0b013e31817e2ec5. [DOI] [PubMed] [Google Scholar]
- 59.Sihler KC, Napolitano LM. Massive transfusion: New insights. Chest. 2009;136:1654–1667. doi: 10.1378/chest.09-0251. [DOI] [PubMed] [Google Scholar]
- 60.Woodruff SI, Dougherty AL, Dye JL, et al. Use of recombinant factor VIIA for control of combat-related haemorrhage. Emerg Med. 2010;27:121–124. doi: 10.1136/emj.2008.060657. [DOI] [PubMed] [Google Scholar]
- 61.Novonordisk. Evaluation of recombinant factor VIIa in patients with severe bleeding (control) Available at: http://clinicaltrials.gov/ct2/show/results/nct00184548.
- 62.CRASH-2 Collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant hemorrhage (CRASH 2): A randomized, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]