Abstract
Pesticide exposure has been implicated as an environmental risk factor for the development of Parkinson’s disease (PD). However, few studies have identified specific pesticides. Previously, we identified elevated serum levels of the organochlorine pesticide β-hexachlorocyclohexane (β-HCH) in PD patients from a small clinical sample. Here, we conducted a case-control study to confirm the association between β-HCH and PD in a larger sample size (n=283) with serum samples of PD patients and controls obtained from UT Southwestern Medical Center and Emory University. Samples were obtained from two discrete periods at both sites, 2001–2003 and 2006–2008, and were analyzed for β-HCH levels. Adjusted odds ratios (ORs) for PD were estimated using logistic regression and generalized estimating equations. The mean serum β-HCH level across all cohorts in this study was 22.3 ng/mg cholesterol (Range: 0 to 376.7), and the levels were significantly higher between samples collected in 2001–2003 vs. 2006–2008. After controlling for age and gender, the OR for increased risk of PD for every 1 ng/mg increase in serum β-HCH ranged from 1.02 – 1.12 across the four different cohorts, and 1.03 (95% CI: 1.00–1.07, p value = 0.031) in the pooled analysis. Furthermore, the OR for increased risk of PD of subjects having serum β-HCH levels above the inter-quartile range of 39.08 ng/mg cholesterol was 2.85 (95% CI: 1.8, 4.48; p value < 0.001). These data are consistent with environmental decreases in β-HCH levels between 2001 and 2008, but they indicate that elevated levels of serum β-HCH are still associated with heightened risk for PD.
Keywords: organochlorine, pesticide, Parkinson’s disease, beta-hexachlorocyclohexane
Introduction
Parkinson disease (PD) can be caused by single gene mutations, but these genetic causes are rare (<10%), suggesting that the causation is more often complex and may involve environmental factors. Epidemiologic studies have identified pesticide exposure as a risk factor (Semchuk et al., 1991; Priyadarshi et al., 2000; Asherio et al., 2006; Kamel et al., 2007; Hancock et al., 2008). However, identification of specific pesticides associated with PD is generally lacking (Elbaz et al., 2009; Gatto et al., 2009; Tanner et al., 2009).
In early attempts to identify pesticides that may be associated with PD, studies with small sample sizes identified an increased presence of organochlorine pesticides in the brains of PD patients (Fleming et al., 1994; Corrigan et al., 1998; Corrigan et al., 2000). Two of these studies reported elevated levels of dieldrin in postmortem PD brains (Fleming et al., 1994; Corrigan et al., 2000), and a recent paper found that serum concentrations of dieldrin were associated with increased risk of PD in samples from Finland taken between 1968 and 1972 (Weisskopf et al., 2010). In addition to dieldrin, elevated levels of the organochlorine pesticide lindane (γ-hexachlorocyclohexane [γ-HCH]) have been found in the substantia nigra of patients with PD vs. controls (Corrigan et al., 2000). More recently, a study from the Faroe Islands reported a small, but significant association between serum levels of β-hexachlorocyclohexane (β-HCH) and increased risk of PD (Petersen et al., 2008). In our previous work, we found that higher levels of serum β-HCH were significantly associated with a greater risk for PD (OR 4.39; 95% CI: 1.67–11.6) in a relatively small patient-derived population (43 controls, 50 PD cases) from the University of Texas Southwestern Medical Center (UTSW; Richardson et al., 2009). Based on the small sample size and the restriction of the samples to one geographic area, the present study was designed to determine the association between β-HCH and PD in a larger sample size (n=283) from two independent locations.
Methods
Study Populations
Control and PD patient samples were obtained from 4 different cohorts, representing 2 time periods at 2 sites. Samples collected from UTSW included samples that were run as part of our previous study (Richardson et al., 2009) and newly collected samples. For the newly collected samples, patients with PD were seen by a neurologist in the Clinical Center for Movement Disorders between March and July, 2008. Pairs of subjects, i.e. a PD patient and a control non-PD partner, were recruited at routine outpatient visits. The inclusion criteria for patients with PD were specific criteria (three of the four features of resting tremor, bradykinesia, rigidity and asymmetric onset, disease duration of three years or more, and absence of atypical features or other causes of parkinsonism (Gelb et al., 1999). Controls were those individuals who accompanied patients to the clinic and were cohabitating companions of the opposite gender and were within five years of the patient’s age, and they did not have any history or exhibit any of the four PD features listed above. Sequential eligible pairs were solicited until there were 26 pairs with male PD patients and 26 pairs with female PD patients. During the recruitment period, only four eligible pairs declined consent, primarily because of fear of phlebotomy or time constraints, and one consenting pair was excluded because the phlebotomist was unable to get an adequate sample from the spouse. In total, there were 40 PD samples and 33 control samples that were collected between 2002 and 2003 from our previous study and 60 PD and 60 control samples collected in 2008. Of the 2008 samples, 50 PD and 50 control samples were newly collected and 10 PD and 10 control samples were part of our previous study that mainly used samples collected between 2001–2003.
For the samples taken at Emory University Medical School, patients were diagnosed by board-certified neurologists that were Movement Disorders specialists at the Movement Disorders Clinic. Diagnostic criteria were the same as described for the UTSW patients. Control samples were part of a clinical research registry in which subjects agreed to provide blood, demographic information, family history, medical history, and cognitive screening (Mini-Mental State Exam and Clock Drawing Test). Many of the subjects also had more intensive assessments through participation in the Emory Alzheimer’s Disease Research Center registry and the NeuroGenetics Research Consortium genetics study. There were 27 PD and 20 control samples that were collected between 2001 and 2003 and 22 PD and 20 control samples collected between 2006 and 2008 from Emory that were included in the analysis. Samples were randomly selected in an attempt to match the gender and age distribution as close to the UTSW sample as possible.
Determination of Pesticide Levels
Serum samples for the early UTSW cohort were obtained as described previously (Richardson et al., 2009). For the new UTSW samples, ten ml of blood was drawn via standard venipuncture into a tube without additives, allowed to sit for 30 min. to allow clotting, and centrifuged at 3000 rpm for 10 minutes. The supernatant was stored at −80°C within 2 hours in 1 ml aliquots. Serum samples from Emory were collected by standard venipuncture techniques and serum isolated and stored at −80°C. All samples were shipped to University of Medicine and Dentistry of New Jersey-Robert Wood Johnson Medical School and analyzed in a blinded fashion with regards to diagnosis.
Serum β-HCH levels were determined by gas chromatography–mass spectrometry (GC/MS) as described previously (Richardson et al., 2009). Based on the limited sample volume available for assay, we were unable to determine total lipid levels in our samples. To address the issue of lipid correction of pesticide levels, we determined the levels of free cholesterol in the serum samples. Free cholesterol in serum was isolated using Solid Phase Extraction (SPE) with a Waters Oasis® (Whaltham MA) 30 mg/ml HLB cartridge mounted in a vacuum manifold, as described previously for organochlorine pesticides. The sorbed pesticides were eluted with 2×1 ml of methylene chloride by gravity flow. To elute sorbed cholesterol, 2ml of acetone was added. The eluent was evaporated under nitrogen to dryness and residue was reconstituted with 100ul methylene chloride. Cholesterol was quantified using a Varian Saturn 2200 GC/MS (Agilent Technologies Palo Alto CA). The GC/MS method was performed using single ion storage (SIS); m/z range 367–382 to analyze extracted free cholesterol.
Statistical Analyses
All analyses were conducted with SAS software, version 9 (SAS Institute, Inc., Cary, NC). The distribution of β-HCH in cases and controls was examined across the 4 different cohorts. Non-parametric analysis of variance (Kruskal-Wallis) for bivariate analysis was used to explore the association between β-HCH, PD, and gender. To assess differences between the two time points, the Wilcoxon signed ranks test was used. Because age and gender were used to match cases and controls in the late UTSW cohort, but not as stringently in the other cohorts, we carried out both conditional and unconditional logistic regression in the UTSW 2008 cohort, which gave similar results. Unconditional logistic regression, controlling for age (years), gender, and location, was used to estimate ORs, and their 95% confidence intervals (CI) for the association between β-HCH and PD diagnosis in the other three cohorts based on per unit increase in cholesterol-corrected β-HCH levels.
Following our initial analyses, we pooled data from the four cohorts to increase the number of samples and determined the odds of having PD per unit increase in β-HCH levels, using generalized estimating equations and the binary distribution. We controlled for age (years) and gender, accounting for covariance structure within each cohort using quasi-likelihood iterative methods (Qaqish and Liang, 1992). Because the enrollment of the cases and controls was carried out at different times and locations, we compared the estimates of the associations from the pooled analysis against that from a meta-analysis. A summary meta-analysis was used to estimate the association between β-HCH and PD among the 4 cohorts using a random-effects model that incorporated both within-study and between-study components of variance (Dersimonian and Laird, 1986). Heterogeneity between the analyses was determined using the Cochran Q test (Higgins and Thompson, 2002).
Because the distribution of β-HCH measures was skewed and β-HCH levels were below detection limits (non-detects) in 156 of 283 participants, we carried out additional log-transformed analyses on the pooled data from the 4 cohorts excluding β-HCH non-detects and an additional analysis excluding samples from the top quintile of β-HCH levels having serum levels over 100 ng/mg cholesterol of β-HCH to determine whether these samples significantly influenced the results. We also computed the OR for PD based on the increase in intraquartile-range β-HCH levels after controlling for age, gender, cohort location, and timepoint (2001–2003 or 2006–2008).
Standard Protocol approvals, registrations, and patient consents
All of the procedures were approved by the Institutional Review boards of UTSW, Emory University, and the University of Medicine and Dentistry of New Jersey–Robert Wood Johnson Medical School and all subjects signed approved consent forms.
Results
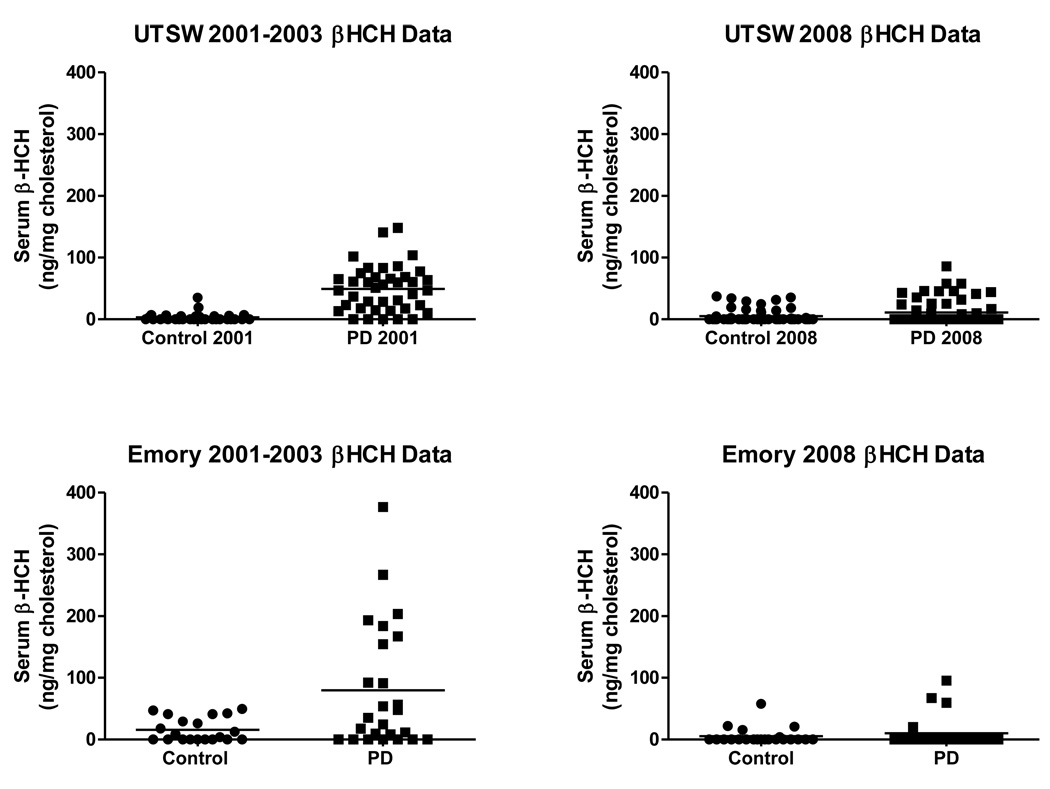
In our initial analysis of the data collected from the UTSW samples, we observed markedly higher levels of serum β-HCH between samples collected in 2001–2003 compared to those collected in 2008. Therefore, we collected additional samples from Emory University spanning these two time periods to provide the four cohorts reported in this study. A total of 283 subjects comprised the four cohorts, with an age range of 47 to 97 years old, (mean age of 68.7 years), and 45 % were women (Table 1). The mean serum β-HCH levels across all cohorts in this study were 22.3 ng/mg cholesterol (Range: 0 – 376.7). Levels of β-HCH were much lower in the cohorts that contained samples taken from 2006–2008 than samples taken from 2001–2003 (p < 0.05). The 2001–2003 Emory cohort had significantly higher levels of β-HCH than the 2001–2003 UTSW (p < 0.05). However, this cohort difference disappeared by the 2006–2008 samples. Levels of β-HCH were significantly higher among PD cases compared to the controls in the two 2001–2003 cohorts (p<0.05). However, the levels of β-HCH were not significantly higher in PD patients vs. controls in the 2006–2008 cohort (Table 2; Fig. 1). There were no gender-related differences in β-HCH levels in any of the cohorts.
Table 1.
Demographic Data for the 4 Cohorts
| Emory | UTSW | |||||||
|---|---|---|---|---|---|---|---|---|
| 2001–2003 | 2006–2008 | 2001–2003 | 2008 | |||||
| Disease status | PD | Control | PD | Control | PD | Control | PD | Control |
| N | 27 | 20 | 22 | 21 | 40 | 33 | 60 | 60 |
| Female | 10 | 10 | 10 | 10 | 11 | 16 | 28 | 32 |
| Male | 17 | 10 | 12 | 11 | 29 | 17 | 32 | 28 |
| Mean Age | 66.5 | 67.3 | 67.1 | 67.6 | 65.7 | 73.3 | 65.9 | 65.3 |
Table 2.
β-HCH Levels in the 4 Cohorts
| Emory | UTSW | |||||||
|---|---|---|---|---|---|---|---|---|
| 2001–2003 | 2006–2008 | 2001–2003 | 2008 | |||||
| β-HCH Levels | Case | Control | Case | Control | Case | Control | Case | Control |
| Mean | 79.43 | 16.92* | 11.18 | 5.71 | 49.31 | 3.27** | 10.77 | 4.39 |
| No. of non-detects | 8 | 8 | 17 | 16 | 5 | 20 | 41 | 41 |
p value <0.001,
p value <0.05 indicates significant differences between cases and control in each cohort, respectively, as determined by the Kruskal-Wallis test.
Figure 1.
Serum levels of β-HCH in samples from controls and PD patients obtained from A. UTSW from 2001–2003 (40 controls, 33 PD cases), B. UTSW in 2008 (60 control, 60 PD cases), C. Emory from 2001–2003 (20 controls, 27 PD cases), and D. Emory from 2006–2008 (21 controls, 22 PD cases). The horizontal bar represents the mean level of β-HCH.
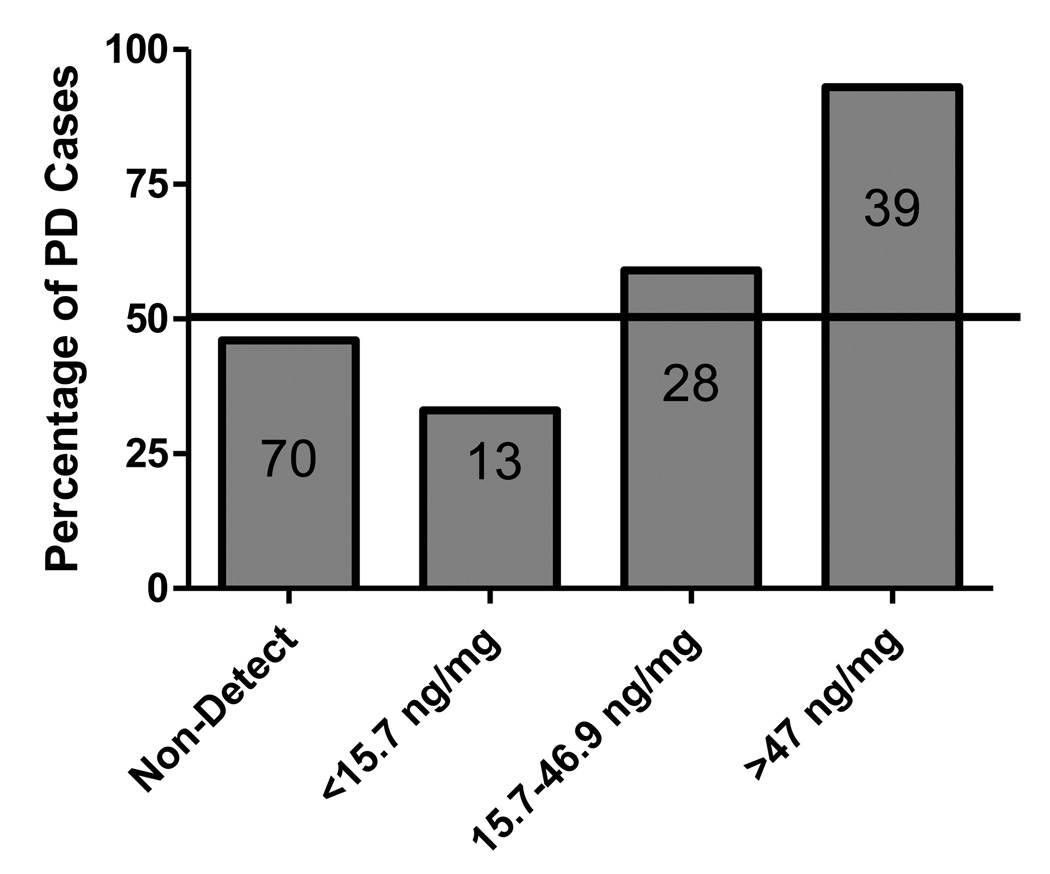
The results of the multivariate analysis of the association between PD and β-HCH levels are presented in Table 3. There was no significant heterogeneity detected across the cohorts (p=0.1178). After controlling for age and gender, the OR for PD for every 1 ng/mg cholesterol increase in β-HCH ranged from 1.02 (p=0.36) to 1.12 (p=0.0016) across the four cohorts, and was 1.03 (95% CI: 1.00–1.07; p value= 0.031) in the pooled analysis. The results remained consistent across all sensitivity analyses and the results of the pooled analysis and the meta-analysis based on per unit increase in β-HCH were remarkably similar and robust, even when non-detects were removed from the analysis (OR= 1.06, 95% CI: 1.02–1.10). Log-transforming the β-HCH levels and removal of all non-detects, did not change the overall association between β-HCH and PD. Because there were only PD cases in the top tertile of β-HCH levels (Fig. 2), we analyzed the data removing the highest values (β-HCH>100, n=15), to be certain that this did not bias the results. Even after removing these cases along with the subjects with non-detectible levels, we still observed a significant increase in risk of PD per unit increase in β-HCH (OR = 1.06, 95% CI: 1.03–1.09; p = 0.0002). The strongest finding was for those subjects with serum β-HCH levels above the inter-quartile range (39.08 ng/mg cholesterol), which were almost three times as likely to be diagnosed with PD (OR = 2.85, 95% CI: 1.8 – 4.48; p < 0.0001).
Table 3.
Association between Parkinson’s disease per unit increase in β-HCH levelsa
| N | OR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| Full Data | |||||
| UTSW | |||||
| 2001–2003 | 73 | 1.12 | 1.04 | 1.20 | 0.0016 |
| 2008 | 120 | 1.03 | 1.00 | 1.06 | 0.034 |
| Emory | |||||
| 2001–2003 | 46 | 1.02 | 1.00 | 1.04 | 0.028 |
| 2006–2008 | 43 | 1.02 | 0.98 | 1.05 | 0.360 |
| bPooled | 282 | 1.03 | 1.00 | 1.07 | 0.031 |
| cMeta-analysis | 282 | 1.03 | 1.01 | 1.06 | 0.009 |
All analyses controlled for age and gender; unit of β-HCH is 1 ng/mg cholesterol.
Pooled analyses accounted for heterogeneity between different cohorts
Meta-analysis estimates were weighted by standard error
Figure 2.
Percentage of PD cases across tertiles of β-HCH Levels. Numbers within the bars represent the number of PD cases in each tertile.
Discussion
Two decades ago, rural living and drinking well water were identified as risk factors for PD, which led to suspicion that pesticide exposure may increase the risk for PD (Koller et al., 1990). An early review of studies concerning pesticide exposure and PD identified 12 of 20 studies that found that pesticide exposure was associated with a 1.6–7 fold increase in risk of PD (Le Couteur et al., 1999). A meta-analysis of case-control studies performed around the same time found an OR of 1.9 for the association between pesticide exposure and development of PD (Priyadarshi et al., 2000). Subsequent epidemiologic studies have generally supported these findings and found that self-reported exposure to pesticides was associated with increased risk of PD (Asherio et al., 2006; Frigerio et al., 2006; Kamel et al., 2007; Tanner et al., 2009). However, researchers have only recently started to explore the relationship between risk of PD and exposure to specific pesticides.
Organochlorine pesticides have been the most frequently implicated class of pesticides with regards to the development of PD (Hatcher et al., 2008). Early studies found that postmortem brain samples from PD patients contained higher concentrations of γ-HCH (lindane), dieldrin and p,p’-DDE than non-PD controls (Fleming et al., 1994; Corrigan et al., 2000). More recently, epidemiological studies have reported significant positive associations between self-reported occupational exposures to organochlorine pesticides and risk of PD (Kamel et al., 2007; Elbaz et al., 2009). Mechanistic studies have demonstrated that organochlorine pesticides are neurotoxic and can damage the dopamine system through generation of oxidative stress, proteasomal dysfunction, disruption of mitochondrial function, and increased alpha-synuclein levels and aggregation, all of which are associated with PD (Kanthasamy et al., 2005; Srivastava and Shivanandappa, 2005; Sun et al., 2005; Hatcher et al., 2008)
Recently, three studies have measured organochlorine pesticide levels in the serum of PD patients and non-PD controls, and found that increasing organochlorine pesticide concentrations in the serum was associated with PD (Petersen et al., 2008; Richardson et al., 2009; Weisskopf et al., 2010). Weisskopf and co-workers (2010) found a significant association (OR per inter-quartile range = 1.28) between increasing serum levels of dieldrin and PD diagnosis in a prospective study of samples from the Finnish Mobile Health Clinic Health Examination taken from 1968–1972. However, this study did not find an association between β-HCH and PD when comparing serum levels from1968–1972 to follow-up cases of PD through 1994. Both Petersen and co-workers (2008) and our previous study (Richardson et al., 2009) found that elevated levels of β-HCH were associated with PD in current patients, with OR of 1.44 and 4.39, respectively. In the present study, we found that the pooled odds of having PD across the four cohorts we studied increased by 2.85 (95% CI: 1.8–4.48; p-value <0.0001) for values above 39.08 ng β-HCH /mg cholesterol, the detectable inter-quartile range in the study population.
β-HCH is a by-product of the manufacturing process of the insecticide γ-HCH (lindane), comprising approximately 10% of technical grade lindane, and a component of the insecticide HCH, which contains a mixture of HCH isomers (ATSDR, 2010). In human studies, higher β-HCH levels have been associated with living in a rural environment, older age, milk consumption, and male gender (Stehr-Green and Lybarger, 1989; Kutz et al., 1991; Becker et al., 2002; Hanaoka et al., 2002), all of which have been identified as risk factors for PD (Chade et al., 2006). Although male gender is a risk factor for PD, we did not find a gender difference in β-HCH levels. This finding is in agreement with data from Weiskopf and co-workers (2010) in samples taken from a much earlier timeframe (1968–1972). However, Petersen and co-workers (2008) found that women had the highest levels of β-HCH and highest risk for PD in samples taken from the Faroe Islands. The reason for this discrepancy is not clear, but may be related to unique dietary factors in the Faroe Islands. For example, increased whale meat and blubber consumption, a major source of β-HCH exposure, in the year previous to the study was noted in the women of this study. However, higher serum concentrations of β-HCH were also found in women over 20 years of age in samples from the CDC’s 4th National Report on Human Exposure to Environmental Chemicals. Unfortunately, the CDC data did not break down the data by age and we have no information on the levels in elderly individuals to compare with our data. Because exposure to β-HCH is most likely through diet (ATSDR, 2010), it is possible that the differences observed are related to differences in dietary composition.
In the present study, we observed similar results for overall risk for PD from the 2 clinical sites. However, the mean serum levels of β-HCH were significantly lower in the most recent samples (2006–2008) compared to those taken at earlier timepoints (2001–2003). This is consistent with studies reporting declining β-HCH levels in various populations over the past three decades (ATSDR, 2010; Radomski et al., 1971; Sturgeon et al., 1998; Link et al., 2005). Thus, the lower levels found in the samples taken between 2006 and 2008 in our study likely reflect continued gradual clearance of β-HCH and diminishing exposure as the result of declining environmental levels. If organochlorine pesticide exposure, and in particular β-HCH or one of the other isomers of HCH, contributed to PD prevalence during the 20th century, it could be hypothesized that there might be a reduced prevalence of cases of PD in the United States that are associated with persistent organochlorine pesticide exposure over time. However, levels of β-HCH and lindane in serum are much higher in other parts of the world where the compounds are still used or were phased out more recently (Bakore et al., 2004; Botella et al., 2004; Weldon et al., 2010), which would suggest that these individuals may be at continued risk. Others that may be at increased risk include people that are highly exposed, i.e. those residing in the Faroe Islands (Petersen et al., 2008), in an arctic Canadian population (Butler et al., 2003), and among people living close to hazardous waste sites contaminated with HCH (Kielb et al., 2010).
Our study has a number of strengths, including a relatively large sample size (149 PD cases and 134 controls) and the use of clinical populations from 2 different sites. The pooled and meta-analysis statistical approaches ensure that the results presented here are not driven by any one subject population studied and the in-depth data analysis indicates that the results are not sensitive to model specification. Finally, the β-HCH levels seen in the control population are similar to those most recently reported by the CDC, which provides support that the control population reflects the exposures seen in the general population of the United States, and suggests that selection bias is unlikely to affect our results. Thus, our results should be generalizable to other elderly populations that are exposed to similar levels of β-HCH.
However, our study does have limitations. As with the other studies that measured pesticides in the serum of PD patients (Petersen et al., 2008; Weisskopf et al., 2010), we were limited to studying organochlorine pesticides that are persistent. Therefore, we cannot rule out the possibility that other non-persistent pesticides may contribute to the development of PD in our cohorts. Because β-HCH accumulates to a greater extent in blood than brain (ATSDR), we cannot rule out the possibility that serum β-HCH is simply a marker for exposure to other persistent pesticides, such as lindane or dieldrin, which accumulate to a greater extent in the brain, and have been found to be elevated in post-mortem PD brain samples (Fleming et al., 1994; Corrigan et al., 2000). Unfortunately, we do not have information on previous exposures that occurred years before diagnosis. Although we accounted for age and gender, which are some of the strongest predictors of PD, there is always a possibility of residual confounding, which could bias the association. A significant confounder that we were unable to control for was smoking status, as we did not have complete information for this variable. However, Weisskopf and co-workers (2010) found that smoking reduced the OR for the association between serum dieldrin levels and PD, suggesting that not having this variable might underestimate the effect size in our study. Thus, additional study is required on potential modifying factors that may affect the association between organochlorine exposure and PD.
In summary, our data support and extend our previous finding that elevated serum β-HCH levels increase the risk of PD. Although we found that the levels of β-HCH have decreased significantly between 2001 and 2008, the association with PD is still significant and the OR is especially strong for those with levels above the inter-quartile range. This suggests that current high levels of serum β-HCH in people over 60 years of age might serve as a useful predictor of PD development.
Acknowledgments
Study Funding: Support provided by the Michael J. Fox Foundation for Parkinson’s Research, the Dallas Area Parkinsonism Society, the Dallas Foundation, the Close to a Cure Foundation, and the National Institutes of Health P30ES0050022, P01ES016731, and P50AG025688.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agency for Toxic Substances and Disease Registry. [Accessed November 1, 2010];Toxicological profile for hexachlorocyclohexane. http://www.atsdr.cdc.gov/toxprofiles/tp43.html.
- Ascherio A, Chen H, Weisskopf MG, O'Reilly E, McCullough ML, Calle EE, et al. Pesticide exposure and risk for Parkinson's disease. Ann Neurol. 2006;60:197–203. doi: 10.1002/ana.20904. [DOI] [PubMed] [Google Scholar]
- Bakore N, John PJ, Bhatnagar P. Organochlorine pesticide residues in wheat and drinking water samples from Jaipur, Rajasthan, India. Environ Monit Assess. 2004;98:381–389. doi: 10.1023/b:emas.0000038197.76047.83. [DOI] [PubMed] [Google Scholar]
- Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M, et al. German Environmental Survey 1998 (GerES III): environmental pollutants in blood of the German population. Int J Hyg Environ Health. 2002;205:297–308. doi: 10.1078/1438-4639-00155. [DOI] [PubMed] [Google Scholar]
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Butler Walker J, Seddon L, McMullen E, Houseman J, Tofflemire K, Corriveau A, et al. Organochlorine levels in maternal and umbilical cord blood plasma in Arctic Canada. Sci Total Environ. 2003;302:27–52. doi: 10.1016/s0048-9697(02)00319-4. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed November 1, 2010];Fourth National Report on Human Exposure to Environmental Chemicals. http://cdc.gov/exposurereport.
- Chade AR, Kasten M, Tanner CM. Nongenetic causes of Parkinson's disease. J Neural Transm Suppl. 2006:147–151. doi: 10.1007/978-3-211-45295-0_23. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Murray L, Wyatt CL, Shore RF. Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson's disease. Exp Neurol. 1998;150:339–342. doi: 10.1006/exnr.1998.6776. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson's disease. J Toxicol Environ Health A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, et al. Professional exposure to pesticides and Parkinson disease. Ann Neurol. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson's disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Frigerio R, Sanft KR, Grossardt BR, Peterson BJ, Elbaz A, Bower JH, et al. Chemical exposures and Parkinson's disease: a population-based case-control study. Mov Disord. 2006;21:1688–1692. doi: 10.1002/mds.21009. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson's disease in rural California. Environ Health Perspect. 2009;117:1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Hanaoka T, Takahashi Y, Kobayashi M, Sasaki S, Usuda M, Okubo S, et al. Residuals of beta-hexachlorocyclohexane, dichlorodiphenyltrichloroethane, and hexachlorobenzene in serum, and relations with consumption of dietary components in rural residents in Japan. Sci Total Environ. 2002;286:119–127. doi: 10.1016/s0048-9697(01)00969-x. [DOI] [PubMed] [Google Scholar]
- Hancock DB, Martin ER, Mayhew GM, Stajich JM, Jewett R, Stacy MA, et al. Pesticide exposure and risk of Parkinson's disease: a family-based case-control study. BMC Neurol. 2008;8:6. doi: 10.1186/1471-2377-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher JM, Pennell KD, Miller GW. Parkinson's disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the Agricultural Health Study. Hum Exp Toxicol. 2007;26:243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- Kamel F, Tanner C, Umbach D, Hoppin J, Alavanja M, Blair A, et al. Pesticide exposure and self-reported Parkinson's disease in the agricultural health study. Am J Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Dieldrin-induced neurotoxicity: relevance to Parkinson's disease pathogenesis. Neurotoxicology. 2005;26:701–719. doi: 10.1016/j.neuro.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Kielb CL, Pantea CI, Gensburg LJ, Jansing RL, Hwang SA, Stark AD, et al. Concentrations of selected organochlorines and chlorobenzenes in the serum of former Love Canal residents, Niagara Falls, New York. Environ Res. 110:220–225. doi: 10.1016/j.envres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Koller W, Vetere-Overfield B, Gray C, Alexander C, Chin T, Dolezal J, et al. Environmental risk factors in Parkinson's disease. Neurology. 1990;40:1218–1221. doi: 10.1212/wnl.40.8.1218. [DOI] [PubMed] [Google Scholar]
- Kutz FW, Wood PH, Bottimore DP. Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue. Rev Environ Contam Toxicol. 1991;120:1–82. doi: 10.1007/978-1-4612-3080-9_1. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, McLean AJ, Taylor MC, Woodham BL, Board PG. Pesticides and Parkinson's disease. Biomed Pharmacother. 1999;53:122–130. doi: 10.1016/S0753-3322(99)80077-8. [DOI] [PubMed] [Google Scholar]
- Link B, Gabrio T, Zoellner I, Piechotowski I, Paepke O, Herrmann T, et al. Biomonitoring of persistent organochlorine pesticides, PCDD/PCDFs and dioxin-like PCBs in blood of children from South West Germany (Baden-Wuerttemberg) from 1993 to 2003. Chemosphere. 2005;58:1185–1201. doi: 10.1016/j.chemosphere.2004.09.061. [DOI] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Bech S, Wermuth L, Weihe P, Nielsen F, et al. Impact of dietary exposure to food contaminants on the risk of Parkinson's disease. Neurotoxicology. 2008;29:584–590. doi: 10.1016/j.neuro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21:435–440. [PubMed] [Google Scholar]
- Qaqish BF, Liang KY. Marginal models for correlated binary responses with multiple classes and multiple levels of nesting. Biometrics. 1992;48:939–950. [PubMed] [Google Scholar]
- Radomski JL, Astolfi E, Deichmann WB, Rey AA. Blood levels of organochlorine pesticides in Argentina: occupationally and nonoccupationally exposed adults, children and newborn infants. Toxicol Appl Pharmacol. 1971;20:186–193. doi: 10.1016/0041-008x(71)90044-5. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Shalat SL, Buckley B, Winnik B, O'Suilleabhain P, Diaz-Arrastia R, et al. Elevated serum pesticide levels and risk of Parkinson disease. Arch Neurol. 2009;66:870–875. doi: 10.1001/archneurol.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson's disease and exposure to rural environmental factors: a population based case-control study. Can J Neurol Sci. 1991;18:279–286. doi: 10.1017/s0317167100031826. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Shivanandappa T. Hexachlorocyclohexane differentially alters the antioxidant status of the brain regions in rat. Toxicology. 2005;214:123–130. doi: 10.1016/j.tox.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Stehr-Green PA, Lybarger JA. Exposure to toxic waste sites: an investigative approach. Public Health Rep. 1989;104:71–74. [PMC free article] [PubMed] [Google Scholar]
- Sturgeon SR, Brock JW, Potischman N, Needham LL, Rothman N, Brinton LA, et al. Serum concentrations of organochlorine compounds and endometrial cancer risk (United States) Cancer Causes Control. 1998;9:417–424. doi: 10.1023/a:1008823802393. [DOI] [PubMed] [Google Scholar]
- Sun F, Anantharam V, Latchoumycandane C, Kanthasamy A, Kanthasamy AG. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther. 2005;315:69–79. doi: 10.1124/jpet.105.084632. [DOI] [PubMed] [Google Scholar]
- Tanner CM, Ross GW, Jewell SA, Hauser RA, Jankovic J, Factor SA, et al. Occupation and risk of parkinsonism: a multicenter case-control study. Arch Neurol. 2009;66:1106–1113. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Knekt P, O'Reilly EJ, Lyytinen J, Reunanen A, Laden F, et al. Persistent organochlorine pesticides in serum and risk of Parkinson disease. Neurology. 2010;74:1055–1061. doi: 10.1212/WNL.0b013e3181d76a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon RH, Webster M, Harley KG, Bradman A, Fenster L, Davis MD. Serum Persistent Organic Pollutants and Duration of Lactation among Mexican-American Women. J Environ Public Health. 2010 doi: 10.1155/2010/861757. 861757. [DOI] [PMC free article] [PubMed] [Google Scholar]