Abstract
Research supports the effectiveness of acupuncture for conditions such as chronic low back and knee pain. In a five-patient pilot study the modality also improved the symptoms of chemotherapy-induced neuropathic pain. Using an established rat model of paclitaxel-induced peripheral neuropathy, we evaluated the effect of electroacupuncture (EA) on paclitaxel-induced hyperalgesia and allodynia that has not been studied in an animal model. We hypothesize that EA would relieve the paclitaxel-induced mechanical allodynia and hyperalgesia, which was assessed 30 minutes after EA using von Frey filaments. Beginning on day 13, the response frequency to von Frey filaments (4-15 g) was significantly increased in paclitaxel-injected rats compared to those injected with vehicle. EA at 10Hz significantly (p<0.05) decreased response frequency at 4-15 g compared to sham EA; EA at 100Hz only decreased response frequency at 15 g stimulation. Compared to sham EA plus vehicle, EA at 10Hz plus either a μ, δ, or κ opioid receptor antagonist did not significantly decrease mechanical response frequency, indicating that all three antagonists blocked EA inhibition of allodynia and hyperalgesia. Since we previously demonstrated that μ and δ but not κ opioid receptors affect EA anti-hyperalgesia in an inflammatory pain model, these data show that EA inhibits pain through different opioid receptors under varying conditions. Our data indicate that EA at 10Hz inhibits mechanical allodynia/hyperalgesia more potently than does EA at 100Hz. Thus, EA significantly inhibits paclitaxel-induced allodynia/hyperalgesia through spinal opioid receptors, and EA may be a useful complementary treatment for neuropathic pain patients.
Keywords: Chemotherapy pain, Acupuncture, Hyperalgesia, Spinal cord, Opioid
1. Introduction
Acupuncture has received increasing public interest since its introduction to the West in the 1970s. In the U.S., approximately one million persons were using the modality annually by 1994 (Paramore, 1997), most commonly for chronic pain. Eight million had used it by 2002 (Barnes P et al., 2004), and three million had used it in the previous 12 months according to a 2007 National Institutes of Health (NIH) survey (Barnes et al., 2008). Acupuncture research has made great progress over the past forty years (Zhao, 2008), supporting the effectiveness of the treatment for conditions such as chronic low back (Manheimer et al., 2005) and knee pain (Kwon et al., 2006).
Chemotherapy-induced peripheral neuropathy (CIPN) is the most common and serious adverse effect of effective chemotherapeutic agents. Paclitaxel-induced neurotoxicity has been reported in 60-70% of all treated patients (Argyriou et al., 2007; Park et al., 2011); it mainly presents as sensory neuropathy, with numbness, tingling, and burning pain being the most common complaints (Berger et al., 1997; Chaudhry et al., 1994; Lipton et al., 1989; Postma et al., 1995; van Gerven et al., 1994). There is no reagent that has robustly been proven effective against symptoms of CIPN (Argyriou et al., 2010). Peripheral pain is the most troublesome symptom of neuropathy. In clinic, acupuncture improved the symptoms of chemotherapy-evoked peripheral neuropathy (Wong and Sagar, 2006). A case report showed acupuncture relieve pain in patients with chemotherapy-evoked peripheral neuropathy (Bao et al., 2011). Animal studies have also shown that electroacupuncture (EA) inhibits hyperalgesia in traumatic nerve injury-induced neuropathic pain animal models (Kim et al., 2004). However, it is not known whether acupuncture relieves hyperalgesia- and allodynia-like behaviors in rats with chemotherapy-evoked peripheral neuropathy. We used an established rat model of paclitaxel-evoked peripheral neuropathy (Flatters and Bennett, 2004; Flatters and Bennett, 2006) to test a hypothesis that EA would relieve the paclitaxel-induced mechanical hyperalgesia- and allodynia-like behavior.
It is well documented that EA’s analgesic effects on acute pain are mediated by the endogenous opioids (Han, 2003). Recent chronic pain acupuncture/EA studies, including our own (Lao et al., 2004), have shown that EA produces anti-hyperalgesia in inflammatory (Yang et al., 2010; Zhang et al., 2002) and traumatic nerve injury-induced neuropathic pain animal models (Kim et al., 2004). Further, it has been demonstrated that spinal μ and δ, but not κ opioid receptors are involved in EA-produced anti-hyperalgesia in complete Freund’s adjuvant (CFA)-(Zhang et al., 2004) and capsaicin (Kim et al., 2009)-induced inflammatory and caudal trunk injury-induced neuropathic pain models (Kim et al., 2004). We hypothesized that spinal opioid receptors are involved differently in EA action on paclitaxel-evoked peripheral neuropathy than in inflammatory pain and traumatic nerve injury-induced neuropathic pain.
2. Results
2.1 EA inhibited paclitaxel-induced hyperalgesia- and allodynia-like behavior
Before paclitaxel injection, mechanical response frequency of the hind paws was the same in all groups of rats (Fig. 1). After drug injection, the response frequencies to bending force stimulation of 4-15 g filaments increased significantly at day 13 compared to those of vehicle-injected rats. Previous studies demonstrate that the mechanical threshold is 15 g in naive rats (Chu et al., 2011; Gong et al., 2010; Obata et al., 2010). Thus we describe paclitaxel-induced responses to 15 g of pressure as hyperalgesia-like behavior and paclitaxel-induced responses to <15 g as allodynia-like behavior.
Fig. 1.
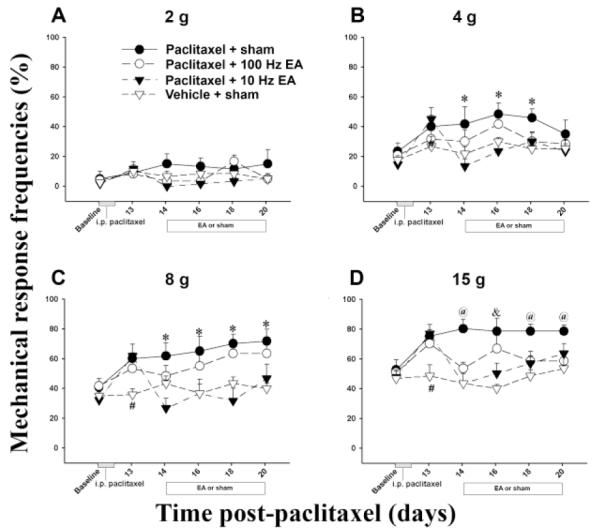
Effect of EA on paclitaxel-induced mechanical allodynia and hyperalgesia (n = 7/group). Mechanical response was evoked with filaments with bending forces of 2 (A), 4 (B), 8 (C), and 15 g (D). From days 13-20 post-paclitaxel injection, drug-injected rats showed significantly higher mechanical response frequencies than did vehicle-injected ones when both groups were given sham EA. Rats given paclitaxel and 10 Hz EA (▼) showed significantly lower mechanical response frequencies to stimulations of 4, 8, and 15 g than did those given paclitaxel plus sham EA (●). Rats given paclitaxel and 100 Hz EA (○) only showed significantly lower mechanical response frequencies to stimulation of 15 g than did control rats that got paclitaxel plus sham EA (●). # P<0.05 vs paclitaxel-injected rats; * P<0.05 vs 10 Hz EA and vehicle-injected rats; & P<0.05 vs 10 Hz EA and vehicle-injected rats; @P<0.05 vs 10 and 100 Hz EA and vehicle-injected rats.
Our data indicate that the paclitaxel induced mechanical hyperalgesia- and allodynia-like behavior. At stimulation of 4, 8, and 15 g, 10 Hz EA treatment significantly (p<0.05) decreased response frequency compared to sham EA while 100 Hz EA treatment only decreased response frequency at 15 g. This indicates that 10 Hz EA inhibited the mechanical hyperalgesia- and allodynia-like behavior more potently than did 100 Hz EA.
2.2 Effect of μ, δ and κ opioid receptor antagonists on EA-produced anti-allodynia and anti-hyperalgesia
We then investigated the mechanisms of 10 Hz EA. To determine the effects of opioid receptor antagonists on 10 Hz EA action, three opioid receptor antagonists, D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-ThrNH2 (CTOP) for μ, naltrindole hydrochloride (NTI) for δ, or nor-Binaltorphimine dihydrochloride (BNI) for κ, were administered i.t. into the spinal cord of animals before EA treatment.
As shown in Figure 2, EA plus vehicle (i.t.) significantly decreased mechanical response frequency compared to sham EA plus vehicle, indicating again that 10 Hz EA inhibits mechanical hyperalgesia- and allodynia-like behavior. EA plus CTOP did not significantly decrease response frequency compared to sham EA plus vehicle, indicating that this antagonist blocked EA-produced pain inhibition. Sham EA plus CTOP did not significantly change mechanical response frequency compared to sham EA plus vehicle, indicating that the antagonist had little effect on the response (Fig. 2).
Fig. 2.
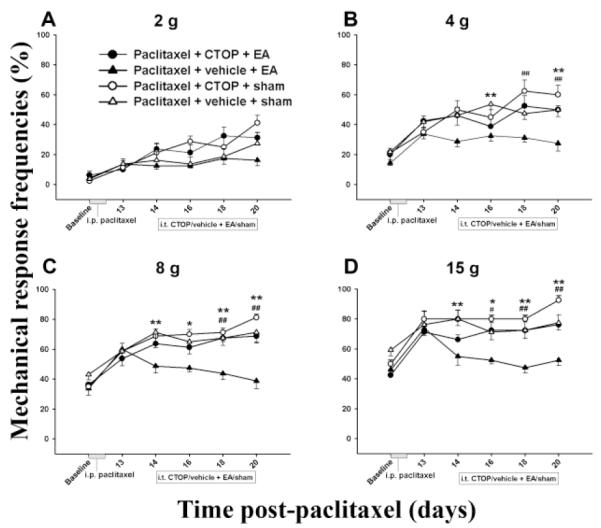
Effect of pretreatment with the μ opioid receptor antagonist CTOP on EA-produced inhibition of hyperalgesia and allodynia in a paclitaxel-induced peripheral neuropathy rat model (n=7 per group). EA plus vehicle significantly decreased mechanical response frequency compared to sham EA plus vehicle. CTOP pretreatment blocked EA-produced inhibition of hyperalgesia and allodynia. * P<0.05 and ** P<0.01 between vehicle + sham (△) and vehicle + 10 Hz EA (▲); # P<0.05 and ## P<0.01 between CTOP + 10 Hz EA (●) vs vehicle + 10 Hz EA (▲).
Similarly, EA plus either the δ or κ opioid receptor antagonist did not significantly decrease mechanical response frequency compared to sham EA plus vehicle, indicating that δ and κ opioid receptor antagonists blocked EA-produced inhibition of mechanical hyperalgesia- and allodynia-like behavior. Sham EA plus NTI or BNI did not significantly change mechanical response frequency compared to sham EA plus vehicle, indicating that these antagonists had little effect on the response (Figs. 3 and 4).
Fig. 3.
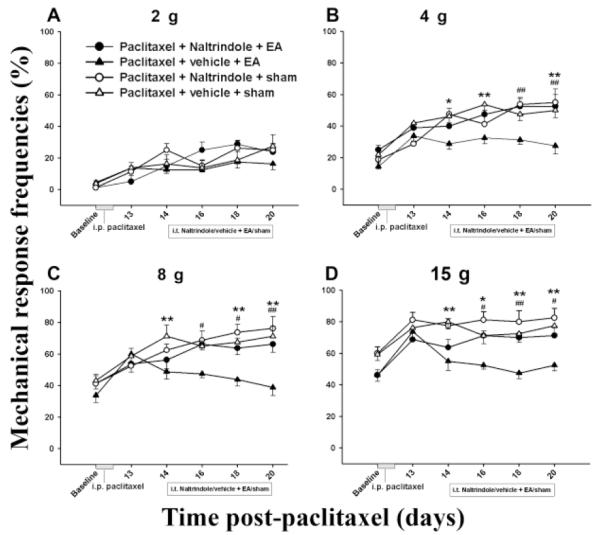
Effect of pretreatment with the δ opioid receptor antagonist NTI on EA-produced inhibition of hyperalgesia and allodynia in a paclitaxel-induced peripheral neuropathy rat model (n=7 per group). EA plus vehicle significantly decreased mechanical response frequency compared to sham EA plus vehicle. NTI pretreatment blocked EA-produced inhibition of hyperalgesia and allodynia. * P<0.05 and ** P<0.01 respectively between vehicle + sham (△) and vehicle + 10 Hz EA (▲); # P<0.05 and ## P<0.01 respectively between NTI + 10 Hz EA (●) vs vehicle + 10 Hz EA (▲).
Fig. 4.
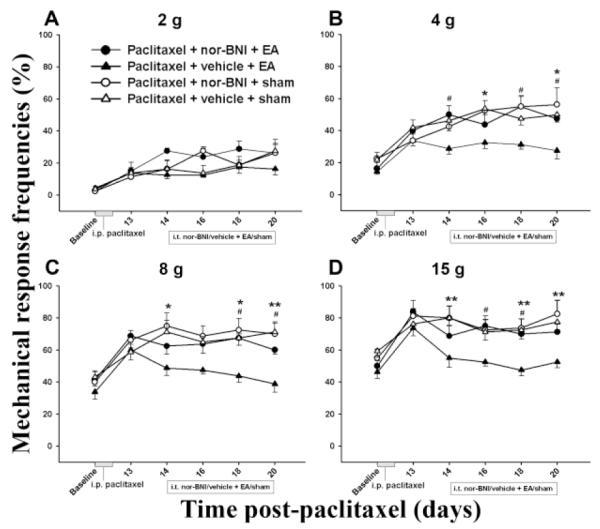
Effect of pretreatment with the κ opioid receptor antagonist BNI on EA-produced inhibition of hyperalgesia and allodynia in a paclitaxel-induced peripheral neuropathy rat model (n=7 per group). EA plus vehicle significantly decreased mechanical response frequency compared to sham EA plus vehicle. BNI pretreatment blocked EA-produced inhibition of hyperalgesia and allodynia. * P<0.05 and ** P<0.01 respectively between vehicle + sham (△) and vehicle + 10 Hz EA (▲); # P<0.05 between BNI + 10 Hz EA (●) vs vehicle + 10 Hz EA (▲).
3. Discussion
The present study demonstrates that 10 Hz EA produces a more potent inhibitory effect than does 100 Hz EA in a paclitaxel-induced neuropathic pain rat model. Similarly, in a previous study, 2 Hz EA induced robust and longer lasting inhibition of mechanical allodynia compared to 100 Hz EA in a caudal trunk nerve injury-induced neuropathic pain model (Kim et al., 2004). It was also found that 2 Hz EA had greater and more prolonged effects on mechanical allodynia and thermal hyperalgesia than did 100 Hz EA in a nerve injury-induced neuropathic pain rat model (Han, 2003; Sun et al., 2002). Thus, lower frequency EA has a potent inhibitory effect on peripheral nerve injury-induced allodynia and hyperalgesia, both metabolic/toxic and traumatic. In a clinical study, acupuncture produced significant improvement in primary and/or secondary symptoms in 77% of patients with diabetic neuropathy, and 67% of the patients were able to stop or reduce their medications significantly (Abuaisha et al., 1998). In HIV-infected individuals, acupuncture significantly reduced the scores for pain/aching/burning, pins and needles, and numbness in the hands and feet (Phillips et al., 2004). In patients with spinal cord injury-induced pain, 24 of 36 showed significant improvement after electroacupuncture treatment (Rapson et al., 2003). Additionally, our unpublished data shows that multiple EA treatments in paclitaxel-injected rats alleviate mechanical hyperalgesia for two to three weeks after the termination of EA treatment. Taken together, these studies show that acupuncture may be useful in the management of neuropathic pain.
The effects of opioid receptor antagonists on acupuncture analgesia could differ depending on conditions. In the present study, all three opioid receptor antagonists blocked EA anti-allodynia and anti-hyperalgesia in paclitaxel-caused neuropathy, indicating that all three opioid receptor subtypes are involved in EA effects on paclitaxel-evoked peripheral neuropathy model. In contrast, our previous study demonstrated that μ and δ but not κ opioid receptors are involved in EA anti-hyperalgesia in a CFA-induced inflammatory pain model (Zhang et al., 2004). It has also been demonstrated that μ and δ but not κ receptors were involved in EA anti-hyperalgesia in a capsaicin-induced inflammatory pain model (Kim et al., 2009) and that EA also inhibited pain through the μ and δ receptors in a caudal trunk injury-induced neuropathic pain model (Kim et al., 2004). These data convincingly show that EA inhibits allodynia and hyperalgesia through different opioid receptors in different situations. The underpinned mechanisms warrant further investigation.
The three opioid receptors, μ, δ and κ, have their own endogenous ligands, endomorphins, enkephalin, and dynorphin, in the spinal cord. These opioids are concentrated in the superficial dorsal horn to exert inhibitory effects on nociceptive transmission (Bodnar and Klein, 2006). Previous studies have demonstrated that EA stimulation frequency determines the type of opioid peptide released in the central nervous system: 2 Hz induces endomorphin and enkephalin; 100 Hz induces dynorphin; 15 Hz induces all three opioids (Han, 2003). Our study showed that the three opioid receptor antagonists blocked EA’s inhibitory effect, leading us to postulate that 10 Hz EA inhibited the paclitaxel-induced neuropathic pain by activating all three opioid receptor subtypes. Since opioid receptors are localized in primary afferent fibers of the spinal cord (Ji et al., 1995), EA-induced opioids may inhibit pain through presynaptic mechanisms (Heinke et al., 2011). In addition, as demonstrated in previous studies, opioid receptors are down-regulated in the spinal cord and dorsal root ganglia in a couple of neuropathic pain animal models (Obara et al., 2009; Takasaki et al., 2006; Zhang et al., 1998). Whether opioid receptors are changed in an animal model of chemotherapy-induced neuropathy has not been investigated and warrants further investigation.
In a study using a DOReGFP reporter mouse, in which a green fluorescent protein (GFP) is covalently bound to a δ opioid receptor (DOR) through a gene suppressant strategy, it was demonstrated that μ and δ opioid receptors are expressed by different subsets of primary afferents and respectively contribute to heat and mechanical pain control (Scherrer et al., 2009). In contrast, a recent study using in situ hybridization, single-cell polymerase chain reaction, and immunostaining demonstrated that δ receptors coexist with μ receptors in small peptidergic dorsal root ganglion neurons. Further, both μ and δ receptor agonists reduce depolarization-induced Ca2+ currents in single small dorsal root ganglion neurons and inhibit afferent C-fiber synaptic transmission in the dorsal spinal cord (Wang et al., 2010). Our data show that both μ and δ opioid receptor antagonists block EA-produced anti-mechanical allodynia and hyperalgesia, suggesting that these two receptors are involved in EA modulation of mechanical pain.
In summary, our experiments show that EA significantly inhibits chemotherapy-induced neuropathic pain through three subtypes of spinal opioid receptors. The results support that EA may be a useful complementary treatment for neuropathic pain patients.
4. Materials and methods
4.1 Animal preparation
Male Sprague Dawley rats (250-270 g body weight, Harlan) were kept under controlled conditions (22°C ± 0.5°C, relative humidity 40-60%, 7:00am to 7:00pm alternate light-dark cycles, food and water ad libitum). The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine. Rats were habituated to a plastic chamber which would be used during the experiments and were handled gently for two 30-minute periods two days before the baseline behavioral test. The paclitaxel-evoked peripheral neuropathy model was produced using an i.p. injection of 2 mg/kg/ml paclitaxel (Taxol®, Bristol–Myers–Squibb) on four alternate days (0, 2, 4, and 6) after baseline measurements of mechanical sensitivity (Flatters and Bennett, 2006). None of the animals treated with paclitaxel showed such signs of ill-health as alopecia, diarrhea, or weight loss, and all gained weight normally. Control rats were produced using i.p. injections of the vehicle, a mixture of 1:2 Cremophor EL/saline, alone on the same time schedule.
4.2 EA treatment
Acupoint GB30 was used. In humans, GB30 is located at the junction of the lateral 1/3 and medial 2/3 of the distance between the greater trochanter and the sacral hiatus; underneath are the sciatic nerve, inferior gluteal nerve and gluteal muscles. Equivalent anatomical landmarks were used to locate these points in the rat. The transposition of an acupoint from the known human map to the anatomically comparable position in animals is widely used to determine points in animals(Lao et al., 2001; Lee and Beitz, 1993; Ma et al., 2005; Zhou et al., 2005) and has been demonstrated to be effective (Lao et al., 2001; Ulett et al., 1998). After cleaning the skin with alcohol swabs, one investigator swiftly inserted two acupuncture needles (gauge # 32, 0.5 inch in length) approximately one-half inch deep into each hind limb of the rat bilaterally at GB 30 while another gently held the animal. The needles were stabilized with adhesive tape (Lao et al., 2004; Zhang et al., 2008). EA (10Hz, 2 mA, 0.4 ms pulse width for 30 minutes) was delivered by a stimulator (Electrostimulator 8-C, Pantheon Research Inc) via two electrodes on days 14, 16, 18 and 20 post-paclitaxel. One end of electrode was soldered to the needle handles in advance, and the other end was connected to the output channel of the stimulator. A symmetrical biphasic wave was delivered to each electrode so that it was alternately positive and negative, and the bilateral needles were stimulated alternately. To minimize discomfort, stimulation intensity was gradually increased over a period of two minutes to 2 mA, which we have found to be the maximum level that can be tolerated by unrestrained rats. During EA treatment, each rat was placed under an inverted clear plastic chamber (approximately 5″x 8″x11″) but was neither restrained nor given any anesthetic. Mild muscle twitching was observed. The animals remained awake and still during treatment and gave no observable signs of distress.
For sham control, acupuncture needles were inserted bilaterally into GB30 without electrical or manual needle manipulation. Sham EA showed little anti-hyperalgesia in our previous study (Lao et al., 2004), making it an appropriate control for non-specific needling effects. Sham- and EA-treated animals were handled identically. EA or sham EA was given once every other day on day 14, 16, 18 and 20 days post-paclitaxel injection. The investigators performing the behavioral tests were blind to treatment assignments.
4.3 Behavioral tests
Mechanical allodynia/hyperalgesia was assessed 30 minutes after each EA treatment using von Frey filaments with bending forces of 2, 4, 6 and 15 g. In ascending order of force, each filament was applied to the mid-plantar area of each hind paw five times, avoiding the base of the tori, with each application held for 5 seconds. Withdrawal responses to the filaments from both hind paws were counted and expressed as the overall percentage response. For example, if a rat withdrew to four out of the total ten von Frey applications, this was recorded as a 40% overall response to that filament.
4.4 I.t. drug delivery
Lumbar punctures were performed as previously described (Li et al., 2011). A PE10 polyethylene tube (Clay Adams) was submerged in 70°C water, stretched to about 150% of its original length to reduce the diameter, and used as an injection catheter. With a 29-gauge needle, another 10-cm PE10 tube was connected to one end of the catheter and then to a 50-μl glass Hamilton syringe with a PE50 tube. The injection catheter was pre-filled with 10 μl of drug or vehicle and 5 μl of saline separated by a small air bubble. Under isoflurane anesthesia, the dorsal pelvic area was shaved and swabbed with 70% alcohol. A 21-gauge sterile needle with the plastic hub removed was inserted between lumbar vertebrae L5 and L6. The catheter was inserted into the guide needle and rostrally advanced 4 cm from the tip of the needle into the lumber enlargement, where its arrival was confirmed by a tail-flick. The drug, or vehicle, was injected and followed by a saline flush. Five minutes after injection the catheter was withdrawn and the needle was removed from the inter-vertebral space.
4.5. Experimental design
Two experiments were conducted: (1) effect of EA on hyperalgesia- and allodynia-like behavior in rats with paclitaxel-evoked peripheral neuropathy and (2) effect of opioid sub-receptor antagonists on EA action.
In Experiment 1, rats were divided into the following four groups (n=7 per group): paclitaxel + 10 Hz EA, paclitaxel + 100 Hz EA, paclitaxel + sham EA, and vehicle + sham EA.
In Experiment 2, paclitaxel-injected rats were randomly divided into the following groups (n=7 per group): (1) intrathecal (i.t.) μ opioid receptor antagonist CTOP (12.5 nmol in 5 μl, Sigma) plus 10 Hz EA; (2) CTOP plus sham EA; (3) i.t. δ opioid receptor antagonist NTI (10 nmol in 5 μl, Sigma) plus 10 Hz EA; (4) NTI plus sham EA; (5) i.t. κ opioid receptor antagonist BNI (10 nmol in 5 μl, Sigma) plus 10 Hz EA; (6) BNI plus sham EA; (7) saline plus 10 Hz EA; and (8) saline plus sham EA. All antagonists were dissolved in saline and administered 10 minutes before each of EA treatment on day 14, 16, 18 and 20 days post-paclitaxel injection. Opiate antagonist dosages are based on previous studies (Kim et al., 2004).
4.6 Statistical analyses
Data from the behavioral tests were presented as mean ± SE and analyzed using repeated measures analysis of variance (ANOVA) followed by Bonferroni multiple comparisons (Graphpad Prism). P<0.05 was set as the level of statistical significance.
Highlights.
Electroacupuncture (EA) may be used as a complementary treatment for chemotherapy-evoked painful peripheral neuropathy.
EA at 10Hz inhibits mechanical allodynia/hyperalgesia more potently than does EA at 100Hz.
Spinal opioids are involved EA inhibition of mechanical allodynia/hyperalgesia.
Acknowledgements
We would like to thank Dr. Lyn Lowry for her editorial support. This work was supported by NIH Grant R21AT004113 and P01 AT002605.
Abbreviation
- EA
electroacupuncture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuaisha BB, Costanzi JB, Boulton AJ, Abuaisha BB, Costanzi JB, Boulton AJ. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long-term study. Diabetes Res. Clin. Pract. 1998;39:115–21. doi: 10.1016/s0168-8227(97)00123-x. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Polychronopoulos P, Koutras A, Xiros N, Petsas T, Argyriou K, Kalofonos HP, Chroni E. Clinical and electrophysiological features of peripheral neuropathy induced by administration of cisplatin plus paclitaxel-based chemotherapy. Eur. J. Cancer Care. 2007;16:231–7. doi: 10.1111/j.1365-2354.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Zolota V, Kyriakopoulou O, Kalofonos HP. Toxic peripheral neuropathy associated with commonly used chemotherapeutic agents. J. BUON. 2010;15:435–46. [PubMed] [Google Scholar]
- Bao T, Zhang RX, Badros A, Lao L. Acupuncture Treatment for Bortezomib-Induced Peripheral Neuropathy: A Case Report. Pain Res. Treatment. 2011 doi: 10.1155/2011/920807. doi:10.1155/2011/920807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. CDC Advance Data Report 2004. 2004:1–20. [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin RL. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007. National Center for Complementary and Alternative Medicine, National Institutes of Health; 2008. [PubMed] [Google Scholar]
- Berger T, Malayeri R, Doppelbauer A, Krajnik G, Huber H, Auff E, Pirker R. Neurological monitoring of neurotoxicity induced by paclitaxel/cisplatin chemotherapy. Eur. J. Cancer. 1997;33:1393–9. doi: 10.1016/s0959-8049(97)00103-2. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Klein GE. Endogenous opiates and behavior: 2005. Peptides. 2006;27:3391–3478. doi: 10.1016/j.peptides.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann. Neurol. 1994;35:304–11. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- Chu LC, Tsaur ML, Lin CS, Hung YC, Wang TY, Chen CC, Cheng JK. Chronic intrathecal infusion of gabapentin prevents nerve ligation-induced pain in rats. Br. J. Anaesth. 2011;106:699–705. doi: 10.1093/bja/aer063. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: Evidence for mitochondrial dysfunction. Pain. 2006;122:245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q-J, Li Y-Y, Xin W-J, Wei X-H, Cui Y, Wang J, Liu Y, Liu C-C, Li Y-Y, Liu X-G. Differential effects of adenosine A1 receptor on pain-related behavior in normal and nerve-injured rats. Brain Res. 2010;1361:23–30. doi: 10.1016/j.brainres.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Han J-S. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends. Neurosci. 2003;26:17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Heinke B, Gingl E, Sandkühler J. Multiple targets of μ-opioid receptormediated presynaptic inhibition at primary afferent Aδ- and C-fibers. J. Neurosci. 2011;31:1313–22. doi: 10.1523/JNEUROSCI.4060-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R, Zhang Q, Law P, Low H, Elde R, Hokfelt T. Expression of mu-, delta-, and kappa-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J. Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Wang J, Lee I, Kim HK, Chung K, Chung JM. Electroacupuncture suppresses capsaicin-induced secondary hyperalgesia through an endogenous spinal opioid mechanism. Pain. 2009;145:332–340. doi: 10.1016/j.pain.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Min B-I, Na HS, Park DS. Relieving effects of electroacupuncture on mechanical allodynia in neuropathic pain model of inferior caudal trunk injury in rat: mediation by spinal opioid receptors. Brain Res. 2004;998:230–236. doi: 10.1016/j.brainres.2003.11.045. [DOI] [PubMed] [Google Scholar]
- Kwon YD, Pittler MH, Ernst E, Kwon YD, Pittler MH, Ernst E. Acupuncture for peripheral joint osteoarthritis: a systematic review and meta-analysis. Rheumatology. 2006;45:1331–7. doi: 10.1093/rheumatology/kel207. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang G, Wei F, Berman BM, Ren K. Electroacupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. J. Pain. 2001;2:111–117. doi: 10.1054/jpai.2001.19575. [DOI] [PubMed] [Google Scholar]
- Lao L, Zhang R-X, Zhang G, Wang X, Berman BM, Ren K. A parametric study of electroacupuncture on persistent hyperalgesia and Fos protein expression in rats. Brain Res. 2004;1020:18–29. doi: 10.1016/j.brainres.2004.01.092. [DOI] [PubMed] [Google Scholar]
- Lee J, Beitz A. The distribution of brain-stem and spinal cord nuclei associated with different frequencies of electroacupuncture analgesia. Pain. 1993;52:11–28. doi: 10.1016/0304-3959(93)90109-3. [DOI] [PubMed] [Google Scholar]
- Li A, Zhang Y, Lao L, Xin J, Ren K, Berman BM, Zhang R-X. Serotonin Receptor 2A/C is Involved in Electroacupuncture Inhibition of Pain in an Osteoarthritis Rat Model. eCAM. 2011 doi: 10.1093/ecam/neq016. doi:10.1093/ecam/neq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Apfel SC, Dutcher JP, Rosenberg R, Kaplan J, Berger A, Einzig AI, Wiernik P, Schaumburg HH. Taxol produces a predominantly sensory neuropathy. Neurology. 1989;39:368–73. doi: 10.1212/wnl.39.3.368. [DOI] [PubMed] [Google Scholar]
- Ma S-X, Ma J, Moise G, Li X-Y. Responses of neuronal nitric oxide synthase expression in the brainstem to electroacupuncture Zusanli (ST 36) in rats. Brain Res. 2005;1037:70–77. doi: 10.1016/j.brainres.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Manheimer E, White A, Berman B, Forys K, Ernst E. Meta-analysis: acupuncture for low back pain. Ann. Intern. Med. 2005;142:651–63. doi: 10.7326/0003-4819-142-8-200504190-00014. [DOI] [PubMed] [Google Scholar]
- Obara I, Parkitna JR, Korostynski M, Makuch W, Kaminska D, Przewlocka B, Przewlocki R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain. 2009;141:283–91. doi: 10.1016/j.pain.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Obata H, Sakurazawa S, Kimura M, Saito S. Activation of astrocytes in the spinal cord contributes to the development of bilateral allodynia after peripheral nerve injury in rats. Brain Res. 2010;1363:72–80. doi: 10.1016/j.brainres.2010.09.105. [DOI] [PubMed] [Google Scholar]
- Paramore LC. Use of alternative therapies: estimates from the 1994 Robert Wood Johnson Foundation National Access to Care Survey. J. Pain Symptom Manage. 1997;13:83–9. doi: 10.1016/s0885-3924(96)00299-0. [DOI] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, Friedlander ML, Lewis CR, Kiernan MC. Early, progressive, and sustained dysfunction of sensory axons underlies paclitaxel-induced neuropathy. Muscle Nerve. 2011;43:367–74. doi: 10.1002/mus.21874. [DOI] [PubMed] [Google Scholar]
- Phillips KD, Skelton WD, Hand GA, Phillips KD, Skelton WD, Hand GA. Effect of acupuncture administered in a group setting on pain and subjective peripheral neuropathy in persons with human immunodeficiency virus disease. J. Altern. Complement. Med. 2004;10:449–55. doi: 10.1089/1075553041323678. [DOI] [PubMed] [Google Scholar]
- Postma TJ, Vermorken JB, Liefting AJ, Pinedo HM, Heimans JJ. Paclitaxel-induced neuropathy. Ann Oncol. 1995;6:489–94. doi: 10.1093/oxfordjournals.annonc.a059220. [DOI] [PubMed] [Google Scholar]
- Rapson LM, Wells N, Pepper J, Majid N, Boon H. Acupuncture as a promising treatment for below-level central neuropathic pain: a retrospective study. J. Spinal Cord Med. 2003;26:21–6. doi: 10.1080/10790268.2003.11753655. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O’Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–59. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun RQ, Wang HC, Wang Y, Luo F, Han JS. Effect of electroacupuncture with different frequencies on neuropathic pain rat model. Chin. J. Appl. Physiol. 2002;18:128–31. [PubMed] [Google Scholar]
- Takasaki I, Nojima H, Shiraki K, Kuraishi Y. Specific down-regulation of spinal mu-opioid receptor and reduced analgesic effects of morphine in mice with postherpetic pain. Eur. J. Pharmacol. 2006;550:62–7. doi: 10.1016/j.ejphar.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Ulett GA, Han S, Han J.-s. Electroacupuncture: mechanisms and clinical application. Biol. Psychiatry. 1998;44:129–138. doi: 10.1016/s0006-3223(97)00394-6. [DOI] [PubMed] [Google Scholar]
- van Gerven JM, Moll JW, van den Bent MJ, Bontenbal M, van der Burg ME, Verweij J, Vecht CJ. Paclitaxel (Taxol) induces cumulative mild neurotoxicity. Eur. J. Cancer. 1994;30A:1074–7. doi: 10.1016/0959-8049(94)90459-6. [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, Lu YJ, Zhang ZN, He SQ, Zheng HC, Wu SX, kfelt TG, Bao L, Zhang X. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc. Natl. Acad. Sci. U S A. 2010;107:13117–22. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Sagar S. Acupuncture treatment for chemotherapy-induced peripheral neuropathy--a case series. Acupunct. Med. 2006;24:87–91. doi: 10.1136/aim.24.2.87. [DOI] [PubMed] [Google Scholar]
- Yang E, Koo S, Kim Y, Lee J, Hwang H, Lee M, Choi S. Contralateral electroacupuncture pretreatment suppresses carrageenan-induced inflammatory pain via the opioid-mu receptor. Rheumatol. Int. 2010;31:725–30. doi: 10.1007/s00296-010-1364-y. [DOI] [PubMed] [Google Scholar]
- Zhang R-X, Lao L, Wang L, Liu B, Wang X, Ren K, Berman BM. Involvement of opioid receptors in electroacupuncture-produced anti-hyperalgesia in rats with peripheral inflammation. Brain Res. 2004;1020:12–17. doi: 10.1016/j.brainres.2004.05.067. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Xin J, Ren K, Qiao JT, Berman BM, Lao L. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur. J. Pain. 2008;12:870–8. doi: 10.1016/j.ejpain.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Shi TJ, Ju G, Elde R, Hokfelt T. Down-regulation of mu-opioid receptors in rat and monkey dorsal root ganglion neurons and spinal cord after peripheral axotomy. Neuroscience. 1998;82:223–40. doi: 10.1016/s0306-4522(97)00240-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y-Q, Ji G-C, Wu G-C, Zhao Z-Q. Excitatory amino acid receptor antagonists and electroacupuncture synergetically inhibit carrageenan-induced behavioral hyperalgesia and spinal fos expression in rats. Pain. 2002;99:525–535. doi: 10.1016/S0304-3959(02)00268-3. [DOI] [PubMed] [Google Scholar]
- Zhao Z. Neural mechanism underlying acupuncture analgesia. Prog. Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Zhou W, Tjen-A-Looi SC, Longhurst JC. Brain Stem Mechanisms Underlying Acupuncture Modality-Related Modulation of Cardiovascular Responses in Rats. J. Appl. Physiol. 2005;99:851–60. doi: 10.1152/japplphysiol.01365.2004. [DOI] [PubMed] [Google Scholar]


