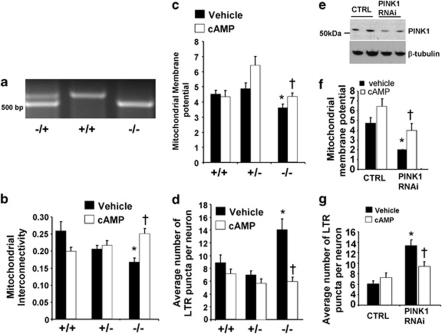
Figure 5.
Treatment with db-cAMP reverses mitochondrial injury and lysosomal expansion induced by loss of endogenous PINK1 in primary cortical neurons. (a) Prenatal E14 mouse pups from mating of PINK1+/− mice were genotyped by PCR amplification. Heterozygous mouse pups show the presence of two bands at 510 and 630 bp, whereas KO pups only show the lower PCR product. (b) Quantification of mitochondrial interconnectivity in wild-type, heterozygous, and PINK1-knockout cortical neurons after 4 h with vehicle or 250 μM db-cAMP (means±S.E. from three independent experiments, 20–25 neurons/condition/experiment; *P<0.05 versus wild-type/vehicle; †P<0.05 versus PINK1 homozygous knockout/vehicle, ANOVA). (c) Quantification of TMRM mitochondrial membrane potential in PINK1-knockout cortical neurons (means±S.E., from two independent experiments, 30–35 neurons/condition; *P<0.05 versus wild-type/vehicle; †P<0.05 versus PINK1 homozygous knockout/vehicle, ANOVA). (d) The average number of LTR-stained lysosomes per neuron (means±S.E. from three independent experiments; *P<0.05 versus wild-type/vehicle; †P<0.05 versus PINK1 homozygous knockout/vehicle, ANOVA). (e) Western blot analysis of endogenous PINK1 levels in cell lysates derived from HT22 cells transiently transfected with PINK1 siRNA for 3 days. (f) Quantification of the mitochondrial membrane potential in cortical neurons transfected with PINK1 siRNA (means±S.E., two independent experiments, 30–35 neurons/condition; *P<0.05 versus control siRNA/vehicle; †P<0.05 versus PINK1 siRNA/vehicle, ANOVA). (g) The average number of lysosomes per cortical neuron treated with control or PINK1 siRNA in the presence or absence of db-cAMP (means±S.E., two independent experiments, 20–25 neurons/condition; *P<0.05 versus control siRNA/vehicle treated; †P<0.05 versus PINK1 siRNA/vehicle treated, ANOVA)