Abstract
The mechanisms implicated in the sympathoexcitation and pressor the response elicited by central CB1R activation are not fully understood. Further, the few reported mechanistic studies on this endeavor were conducted in anesthetized rats. Therefore, it was important to identify the dose-related cardiovascular responses elicited by central administration of the cannabinoid receptor (CB1R) agonist WIN55,212-2 in conscious rats. The second and main objective of the study was to test the hypothesis that brainstem GABAergic transmission is implicated in the CB1R-evoked sympathoexcitation/pressor response. In conscious rats, intracisternal (i.c) WIN55,212-2 (3, 10, 30 μg/rat) elicited dose-dependent increases in mean arterial pressure (MAP) and plasma norepinephrine (NE; index of sympathoexcitation), and reduced heart rate (HR). Subsequent neurochemical studies showed that i.c WIN55,212-2 (15 μg/rat) significantly increased the number and percentage of neurons that exhibited dual immunostaining for tyrosine hydroxylase (catecholaminergic neurons) and c-Fos (marker of neuronal activity) within the rostral ventrolateral medulla, which suggests enhanced central sympathetic tone. These neurochemical responses along with the increases in MAP and plasma NE were drastically attenuated by prior: (i) blockade of central CB1R by i.c AM251 (30 μg/rat) or (ii) activation of central GABAAR by i.c muscimol (0.1 μg/rat). Collectively, these neurochemical and cardiovascular findings are the first to suggest a pivotal role for the inhibition of brainstem GABAergic transmission in the central CB1R-evoked sympathoexcitation/pressor responses in conscious rats.
Keywords: blood pressure, cannabinoids, CB1R, brainstem, GABAAR, muscimol
1. Introduction
Cannabinoids elicit complex cardiovascular responses via peripheral and central cannabinoid receptors (Gardiner et al., 2002; Malinowska et al., 2010; Niederhoffer et al., 2003; O’Sullivan et al., 2007; Pfitzer et al., 2004; Seagard et al., 2004; Wheal et al., 2007). In conscious rats, systemic cannabinoids, e.g. anandamide or WIN55,212-2, cause pressor and bradycardic responses (Gardiner et al., 2001; Stein et al., 1996), which mimic reported responses in humans (Benowitz et al., 1979; Foltin et al., 1987; Sidney, 2002). The pressor response elicited by systemic WIN55,212-2 in conscious animals was attenuated by ganglion blockade, providing evidence for a central site of action (Gardiner et al., 2001). Notably, the pressor response elicited by central CB1R activation seems to involve neurons that control sympathetic activity in the rostral ventrolateral medulla (RVLM) because: (i) the CB1R agonists WIN55,212-2 or CP-55940 increased sympathetic nerve activity, plasma norepinephrine and blood pressure in conscious rabbits (Niederhoffer and Szabo, 2000) and in anesthetized rats (Pfitzer et al., 2004); these responses were attenuated by pretreatment with the CB1R antagonist SR171416A; (ii) microinjection of WIN55,212-2 into the RVLM elicited a pressor response and enhanced sympathetic nerve activity (Padley et al., 2003). However, the mechanism by which the CB1R activation induces central sympathoexcitation is not fully known.
In the CNS, endocannabinoids modulate the release of both inhibitory (GABA) and excitatory (glutamate) neurotransmitters (Kreitzer and Regehr, 2001; Ohno-Shosaku et al., 2001; Vaughan et al., 1999). Pertinent to the current study, in cultured neurons from the rostral ventromedial medulla (RVM), CB1R activation inhibited GABA release (Vaughan et al., 1999). This in vitro finding highlighted the unexplored possibility that inhibition of brainstem GABAergic input, which exerts tonic restraining influence on RVLM neuronal activity (Amano and Kubo, 1993; Menezes and Fontes, 2007), might underlie the CB1R-evoked sympathoexcitation/pressor response (Padley et al., 2003).
The first objective of the present study was the elucidation of the cardiovascular responses elicited by central CB1R activation in a conscious rat model as none of the reported findings with the CB1R agonist WIN55,212-2 were generated in conscious rats. To this end, we investigated the dose-related effects of intracisternal WIN55,212-2 on blood pressure, heart rate, and plasma NE (index of sympathetic activity) (Hubbard et al., 1986; Pfitzer et al., 2004) in conscious unrestrained rats. Further, we utilized dual labeling immunofluorescence to measure (via c-Fos protein; c-Fos-ir) the activity of catecholaminergic (tyrosine hydroxylase immunoreactive neurons, TH-ir) neurons in the RVLM following central CB1R activation in the absence or presence of selective CB1R blockade (AM251). Finally, we adopted a pharmacological approach to test our hypothesis that inhibition of central GABAergic signaling accounts, at least partly, for the central CB1R-evoked sympathoexcitation/pressor response. This pharmacological approach is based on the premise that attenuation of sympathoexcitation/pressor response elicited by angiotensin II or insulin, by prior activation of central GABAAR (muscimol), suggests that inhibition of GABAergic signaling mediates these responses (Unger et al., 1983; Ward et al., 2011). Therefore, we investigated the effect of muscimol pretreatment on the cardiovascular, biochemical and neurochemical responses elicited by central CB1R activation in conscious rats.
2. Results
2.1. WIN55,212-2 evoked dose-related increases in MAP and plasma NE in conscious rats
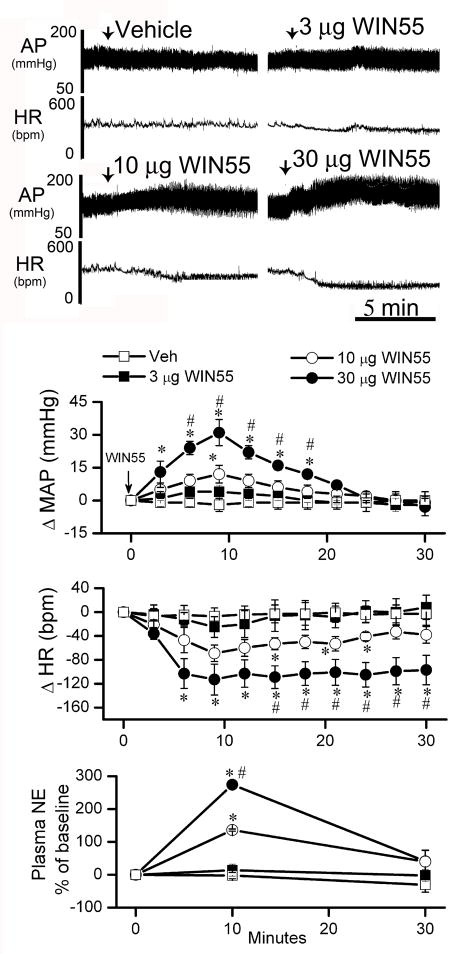
Intracisternal administration of reported doses of WIN55,212-2 in studies conducted in conscious rabbits (Niederhoffer and Szabo, 2000) or anaesthetized rats (Pfitzer et al., 2004) failed to change MAP in conscious unrestrained rats (data not shown). In a subsequent preliminary study, we established a dose range of i.c WIN55,212-2, which elicited dose-related increases in BP and plasma NE along with bradycardia in conscious rats (n=4). Each animal received 5 μl vehicle and 3 doses of WIN55,212-2 (3, 10, 30 μg/rat; i.c) on 2 separate days. On day 1, animals received vehicle and 3 μg WIN55,212-2, and on day 2, they received 10 and 30 μg WIN55,212-2. Blood samples, for plasma NE measurement, were collected before, and 10 and 30 min after each injection. An equal volume of saline was injected after the collection of each blood sample. WIN55,212-2 injections were separated by 1 hr to allow full recovery from the effects produced by the previous dose. Baseline MAP and HR were similar in all groups used in the study (Table 1). Basal plasma NE value (prior to vehicle injection) was 980 ± 77 pg/ml, (n=4). The pressor effect of WIN55,212-2 peaked at approximately 10 min, and subsided by 30 min (Fig. 1). Similarly, a significant (P<0.05) increase in plasma NE, which paralleled the pressor response, peaked at 10 min and subsided by 30 min (Fig. 1). On the other hand, the bradycardic response was immediate and lasted longer than the increases in MAP and plasma NE (Fig. 1).
Table 1.
Baseline MAP (mmHg) and HR (bpm) values before and after pretreatment (preceding i.c WIN55,212-2 or vehicle, when applicable). Values are means ± S.E.M
| Treatment | n | MAP
|
HR
|
||
|---|---|---|---|---|---|
| before | after | before | after | ||
| Vehicle | 4 | 115.0±6.0 | ------ | 395±12 | ----- |
| 3 μg WIN55,212-2 | 4 | 111.3±3.0 | ------ | 375±10 | ----- |
| 10 μg WIN55,212-2 | 4 | 108 ± 6.0 | ------ | 360± 11 | ----- |
| 30 μg WIN55,212-2 | 4 | 112.0±6.0 | ------ | 356±15 | ----- |
| Vehicle + 15 μg WIN55,212-2 | 6 | 111.3±3.0 | 112.0±3.0 | 375±10 | 363±13 |
| AM251 + Vehicle | 5 | 113.0 ±4.0 | 110.0 ±4.0 | 398±16 | 390±20 |
| AM251 + 15 μg WIN55,212-2 | 8 | 108.0±2.0 | 113.0±5.0 | 400±16 | 410±18 |
| Vehicle + 15 μg WIN55,212-2 | 5 | 108.3±6.0 | 110.0±3.0 | 365±10 | 360±16 |
| Muscimol + Vehicle | 5 | 110.0±4.0 | 103.0±4.0 | 440±12 | 400±12 |
| Muscimol + 15 μg WIN55,212-2 | 8 | 106.0±2.0 | 99.0±5.0 | 450±16 | 410±18 |
Figure 1. Central WIN55,212-2 dose-related hemodynamic changes in conscious rats.
Representative tracings and time course changes in mean arterial pressure (ΔMAP), heart rate (ΔHR) and percent change of plasma NE from baseline evoked by intracisternal WIN55,212-2 (WIN55; 3, 10 and 30 μg/rat) or its vehicle (veh) in conscious rats. On day 1, the vehicle and the 3 μg dose were administered and the 10 and 30 μg were administered on day 2. One h was allowed between consecutive WIN55,212-2 doses to permit BP and HR to return to baseline values. Arrow marks WIN55,212-2 injection. Values are mean ± S.E.M. (n=4 in each group). * or # P<0.05 significantly different compared to vehicle (veh) or low dose WIN55,212-2 (3 μg WIN55) values, respectively.
2.2. Cardiovascular, biochemical and neurochemical effects of i.c WIN55,212-2 in the presence or absence of central CB1R blockade or GABAAR stimulation
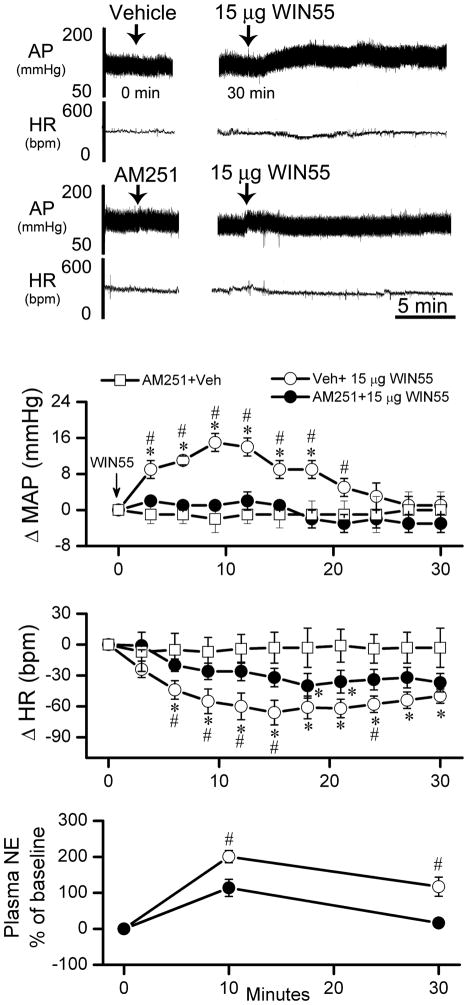
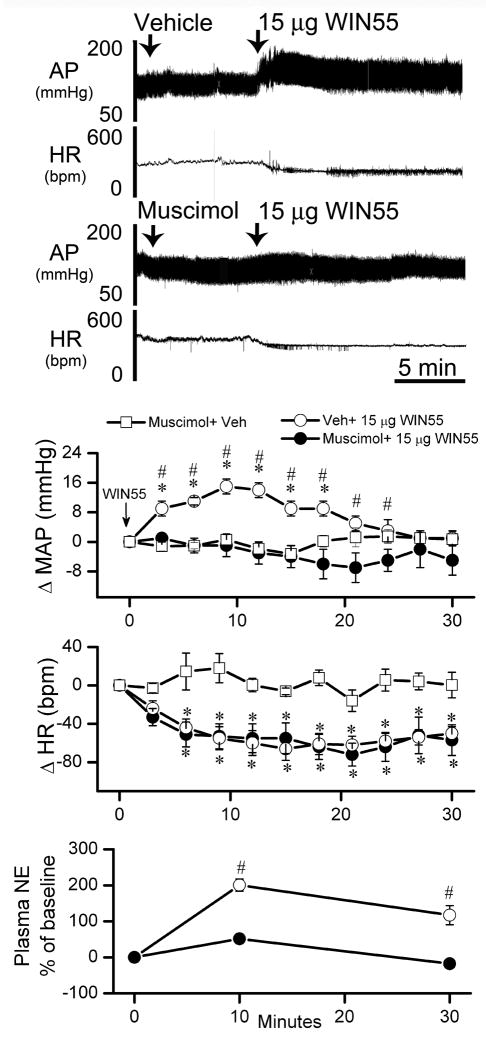
The first objective of this experiment was to verify the involvement of brainstem CB1R in the WIN55,212-2 evoked increases in MAP, plasma NE. Additionally, we investigated whether activation of the TH-ir neurons in the RVLM is implicated in central CB1R-evoked increases in MAP and plasma NE. A dose of WIN55, 212-2 (15 μg/rat, i.c), selected from the dose range discussed above, produced MAP, HR and plasma NE responses that fell between those produced by the 10 μg and the 30 μg doses (Figs. 1 and 2). Pretreatment, 30 min earlier, with the CB1R antagonist AM251 (30 μg/rat, i.c), which did not alter the measured variables (Table 1 and Fig. 2), significantly (P<0.05) attenuated WIN55,212-2 (15 μg/rat, i.c)-evoked increases in MAP and plasma NE as well as the bradycardic response (Fig. 2). Similarly, the GABAA receptor agonist muscimol (0.1 μg/rat, i.c) had no significant effect on blood pressure or heart rate (Table 1). As shown in Fig. 3, pretreatment with muscimol (0.1 μg/rat, i.c) abrogated the increases in BP and plasma NE levels, but not the bradycardia, induced by WIN55,212-2 (15 μg/rat, i.c). Prior to WIN55,212-2 injection, basal plasma NE measured in the group that received the vehicle (975 ± 90 pg/ml; n=6) was not significantly different from the values obtained in rats pretreated with AM251 (1170 ± 65 pg/ml; n=13). Also, plasma NE values following muscimol (910 ± 85 pg/ml; n=13) or its vehicle (965 ± 50 pg/ml; n=5) were not significantly different. Plasma NE values in Figs. 1–3 were calculated as percent change from basal values after pretreatment, and prior to WIN55,212-2 or its vehicle injections.
Figure 2. Hemodynamic effects of i.c WIN55,212-2 in presence or absence of the CB1R antagonist AM251.
Representative tracings and time course changes in mean arterial pressure (ΔMAP), heart rate (ΔHR) and percent change of plasma NE from baseline evoked by intracisternal WIN55,212-2 (Veh +15 μg WIN55) or vehicle, indicated by arrow (top panel), in conscious rats 30 min after i.c pretreatment with 30 μg/rat of the selective CB1R antagonist AM251 (AM251 + 15 μg WIN55) or its vehicle (AM251 + Veh). Values are mean ± S.E.M. of 4 to 8 observations. * or # P < 0.05 versus respective “AM251 + Veh” or “AM251 + 15 μg WIN” values, respectively.
Figure 3. Brainstem GABAA receptor activation attenuates central CB1R-evoked increases in BP and plasma NE.
Representative tracings and time course changes in mean arterial pressure (ΔMAP), heart rate (ΔHR) and percent change of plasma NE evoked by i.c WIN55,212-2 (Veh + 15 μg WIN55) or its vehicle, indicated by arrow (top panel), in conscious rats intracisternally pretreated, 10 min earlier, with 0.1 μg/rat of the selective GABAAR agonist muscimol (Muscimol + 15 μg WIN55) or its vehicle (Muscimol + Veh). Values are mean ± S.E.M. of 6 to 8 observations. * or # P < 0.05 versus respective “Muscimol+ Veh” or “Muscimol+15 μg WIN55” values, respectively.
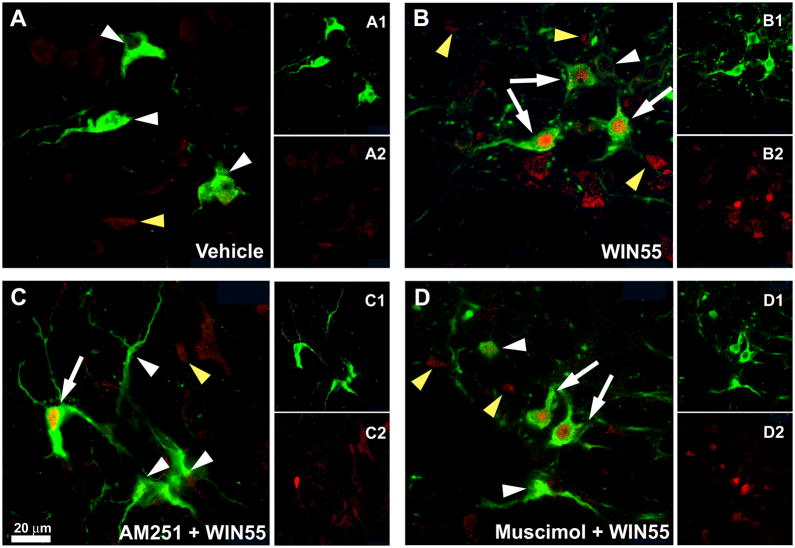
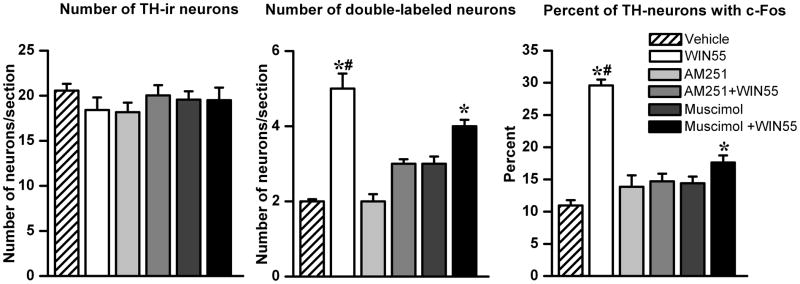
In a parallel experiment, in which c-Fos expression was quantified in the RVLM neurons, WIN55,212-2 (15 μg/rat, i.c), compared to control (vehicle), significantly (P<0.05) increased the percentage of RVLM neurons with dual immunostaining for TH-ir and c-Fos (Figs. 4 and 5). AM251 (30 μg/rat, i.c) or muscimol (0.1 μg/rat, i.c) did not significantly alter the basal TH-ir/c-Fos ratio but significantly (P<0.05) attenuated WIN55,212-2 evoked increases in the number and percentage of RVLM neurons that exhibited dual immunostaining for TH and c-Fos. As shown in Fig. 5, the total number of TH-ir neurons in the RVLM was similar in all groups.
Figure 4. Dual-labeling immunofluorescence photomicrographs depicting CB1R-induced c-Fos expression in catecholaminergic neurons in the RVLM.
Confocal dual-channel images showing tyrosine hydroxylase immunoreactive (TH-ir) neurons (green) and c-Fos immunoreactive (Fos-ir) cell nuclei (red) in RVLM of rats treated as described under methods with (A) vehicle, (B) 15 μg WIN55,212-2, (C) AM251 + 15 μg WIN55,212-2 and (D) Muscimol + 15 μg WIN55,212-2. White or yellow arrowheads indicate single labeled TH-ir neurons or c-Fos-ir cell nuclei, respectively. White arrows denote c-Fos/TH co-labeled cells. Scale bar, 20 μm.
Figure 5. Quantitative analysis of CB1R-induced c-Fos expression in RVLM catecholaminergic neurons.
Number of TH-ir neurons, c-Fos/TH dual-labeled neurons and percentage of TH-ir neurons colocalized with c-Fos in the RVLM of rats treated, as described under methods, with either vehicle, WIN55,212-2 (i.c; 15 μg/rat), AM251 (i.c; 30 μg/rat), AM251 prior to WIN55,212-2, muscimol (i.c; 0.1 μg/rat) or muscimol prior to WIN55,212-2. Bar graphs represent mean ± S.E.M. of data obtained from 4–6 coronal brainstem sections/animal (n=3–5 rats/group) using one-way ANOVA followed by Bonferroni comparison test. * or # P <0.05 compared to either vehicle or all other treatments values, respectively.
3. Discussion
The molecular mechanisms that underlie the sympathoexcitatory/pressor response elicited by central CB1R activation are not fully delineated. The most important findings of this study are: (i) i.c. WIN55,212-2, a non-selective CB1-CB2 receptor agonist, elicited dose-dependent increases in blood pressure and plasma NE along with bradycardic response in conscious rats; (ii) i.c WIN55,212-2 enhanced RVLM-catecholaminergic neuron activity (c-Fos); (iii) the neurochemical, biochemical and cardiovascular responses evoked by WIN55,212-2 were drastically reduced by prior central CB1R blockade (AM251) or activation of central GABAAR (muscimol). Collectively, these novel findings suggest a pivotal role for local (RVLM) and/or GABAergic input from other brain regions in the central CB1R-evoked sympathoexcitation/pressor response in conscious rats.
We show, for the first time, that in conscious rats, i.c WIN55,212-2 elicited dose-dependent increases in MAP and plasma NE levels and reductions in HR. While the pressor response peaked at approx.10 min and disappeared by 30 min, the bradycardic response was immediate and lasted beyond the 30 min recording time (Fig. 1). The bradycardic response has been shown to be central in origin and vagally mediated because it was absent following atropine or vagotomy (Niederhoffer and Szabo, 1999; Niederhoffer and Szabo, 2000). Our studies were conducted in conscious rats, to circumvent the negative impact of anesthesia, which dramatically compromises cannabinoid evoked hemodynamic responses (Gardiner et al., 2001; Lake et al., 1997; Stein et al., 1996). Notably, the pressor response elicited by central WIN55,212-2 administration fully agrees with reported findings in experimental animals (Niederhoffer and Szabo, 1999; Niederhoffer and Szabo, 2000; Pfitzer et al., 2004), and replicates a similar response in humans (Benowitz et al., 1979; Foltin et al., 1987; Sidney, 2002). WIN55,212-2 doses used in the present study were relatively higher than those used in anesthetized rats (Pfitzer et al., 2004). This may be attributed to: (i) the use of conscious rats in the present study, which exhibit a higher sympathetic tone compared to anesthetized animals and/or (ii) we allowed longer time, 1 h vs. only 20 min, between consecutive WIN55,212-2 doses, which may have resulted in cumulative responses in reported studies (Pfitzer et al., 2004).
It was important to confirm the involvement of CB1R in the pressor response elicited by WIN55,212-2 in our model system for two reasons. First, ours are the first findings to demonstrate the WIN55,212-12 evoked pressor/sympathoexcitation in conscious rats. Second, this endeavor necessitated the use of higher doses, than those reported in other species, of WIN55,212-2, which is a mixed CB1R/CB2R agonist (Griffin et al., 1998; Showalter et al., 1996). The ability of the selective CB1R antagonist AM251 (Niederhoffer and Szabo, 1999; Niederhoffer and Szabo, 2000; Pfitzer et al., 2004) to significantly reduce WIN55,212-2 evoked elevations in BP and plasma NE supports the involvement of central CB1R in the observed responses in our model system.
A second objective of the current study was to elucidate the potential role of the RVLM neurons in the central CB1R-evoked sympathoexcitation (increased plasma NE) and pressor response. We present the first in vivo evidence that implicates, at least partly, the activation of RVLM catecholaminergic neurons in the central WIN55,212-2 evoked elevations in MAP and plasma NE. Importantly, the significant increase in the percentage of catecholaminergic neurons (TH-ir) expressing c-Fos (Figs. 4 and 5), paralleled the increases in BP and plasma NE and such increases were reduced by prior blockade of central CB1R (AM251); AM251 alone had no effect on any of the measured variables (Figs 2 and 5). Collectively, these neurochemical and cardiovascular findings with i.c WIN55,212-2 in absence or presence of AM251 support the dependence of the increases in BP and plasma NE, at least partly, on central CB1R-mediated increase in RVLM neuronal activity. These findings fully agree with previously reported studies that microinjection of WIN55,212-2 into the RVLM elicited a pressor response and enhanced sympathetic nerve activity (Padley et al., 2003). Further, these findings suggest that central CB1R does not tonically influence baseline sympathetic activity or BP in our model system, the conscious unrestrained rat.
We hypothesized that the increase in RVLM catecholaminergic neuron activity was caused by central CB1R mediated inhibition of local (RVLM) and/or GABAergic input from other brain regions because: (i) CB1R activation modulates excitatory (glutamate) and inhibitory (GABA) neurotransmission in the CNS (Drew et al., 2008; Freund et al., 2003; Jelsing et al., 2008; Padley et al., 2003; Pilowsky and Goodchild, 2002; Piomelli, 2003); (ii) GABAergic input tonically inhibits RVLM neuronal activity (Amano and Kubo, 1993; Menezes and Fontes, 2007) and microinjection of muscimol into the RVLM elicits hypotensive response (Menezes and Fontes, 2007); (iii) CB1R activation causes inhibition of GABAergic transmission in cultured RVM neurons (Vaughan et al., 1999). To test our hypothesis, we adopted a reported pharmacological approach in which the selective GABAAR agonist muscimol was injected intracerebroventricularly or microinjected into neuronal pools that project to the RVLM and proved that the sympathoexcitation and pressor responses caused by angiotensin II or insulin was mediated by the inhibition of central GABAergic neurotransmission and involves the RVLM (Unger et al., 1983; Ward et al., 2011). In full agreement with this reported premise, are our novel findings that intracisternal pretreatment with muscimol reduced the WIN55,212-2 evoked neurochemical and pressor responses (Figs. 3–5). Notably, muscimol did not affect the central CB1R-evoked bradycardia (Fig. 3). This might be explained, at least partly, by the dependence of the CB1R-mediated bradycardia on the increase in vagal tone (Niederhoffer and Szabo, 1999; Niederhoffer and Szabo, 2000). In support of this notion was the attenuation by AM251 of the CB1R-mediated bradycardia (Fig. 2). These findings raise the interesting possibility that GABAergic neurotransmission does not contribute significantly to the vagal control of heart rate following central CB1R activation although it remains to be determined if higher doses of muscimol could attenuate the central CB1R-mediated bradycardia. It is imperative to note, however, that higher doses of muscimol were avoided in the present and reported studies because they elicit hypotension, bradycardia and marked behavioral responses (sedation) that could confound data interpretation (Unger et al., 1983). Notably, as was the case in the latter study with i.c.v muscimol, i.c administration of the same dose of muscimol in the present study had no significant effect on BP in conscious rats. Collectively, these findings implicate inhibition of central GABAergic signaling in the CB1R-mediated neurochemical responses in the RVLM and the subsequent pressor response. Nonetheless, our findings do not preclude the involvement of other brain structures in the observed responses since the drugs were administered intracisternally.
In summary, the present study is the first to establish a causal link between brainstem GABAergic neurotransmission and the central CB1R-evoked sympathoexcitatory/pressor responses. This central CB1R mediated inhibition of the restraining influence of GABAergic neurotransmission, mediated at the RVLM level and/or via input from other brain regions, may unleash endogenous neuronal activators or causes imbalance between excitatory and inhibitory neurotransmitters, which ultimately explains the CB1R-evoked activation of the catecholaminergic (tyrosine hydroxylase expressing) neurons in the RVLM. This central sympathoexcitation is expected to lead to the higher plasma NE and blood pressure, which accompanied the neurochemical and pressor responses, in the present study. The present findings are clinically relevant because they replicate a similar CB1R-evoked blood pressure response in humans and were observed in conscious unrestrained rats in the absence of any confounding effects of anesthetics. The molecular mechanisms that link central CB1R signaling to the central GABAergic neurotransmission remain to be elucidated.
4. Materials and Methods
Male Sprague-Dawley rats (300–350 g, Charles River, Raleigh, NC) were housed two per cage in a room with controlled environment at a constant temperature of 23 ± 1°C, humidity of 50% ± 10% and a 12:12-h light/dark cycle. Food (Prolab Rodent Chow, Prolab RMH 3000; Granville Milling, Creedmoor, NC) and water were provided ad libitum. All surgical, experimental, and animal care procedures were performed in accordance with, and approved by, the Institutional Animal Care and Use Committee and in accordance with the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals.
4.1. Intra-arterial and intracisternal (i.c) cannulation
These surgeries were performed as reported in our previous studies (Nassar and Abdel-Rahman, 2008). Briefly, 5 days before the experiment, rats were anaesthetized with ketamine (9 mg/100 g) and xylazine (1 mg/100 g, i.p) and a polyethylene catheter (PE50 connected to PE10) was placed in the abdominal aorta via the femoral artery for blood pressure measurement. For i.c drug administration, a stainless steel guide cannula (23G; Small Parts, Miami, FL) was implanted into the cisterna magna. The guide cannula was passed between the occipital and the cerebellum through a hole drilled 1 to 1.5 mm distal to the caudal edge of the occipital bone so that the guide cannula tip protrudes into the cisterna magna. The cannula was secured in place with small metal screws and dental acrylic cement (Durelon; Thompson Dental Supply, Raleigh, NC). The patency of the guide cannula was verified when a spontaneous flow of cerebrospinal fluid was observed and by gross post-mortem histological verification after routine injection of 2 μL of fast green dye (EM Sciences, Cherry Hill, NJ) at the end of the experiment.
4.2. Blood pressure and heart rate measurements
On the day of the experiment, the arterial catheter was flushed with heparinized saline (100 IU/ml) and connected to a Gould-Statham pressure transducer (Oxnard-CA). BP was recorded by ML870 (PowerLab 8/30), and analyzed using LabChart (v.6) pro software (ADInstruments, Colorado Spring, CO). Heart rate was extracted from BP recording using the LabChart (v.6) blood pressure analysis module and both variables were continuously recorded and stored for offline analysis. BP and HR were allowed to stabilize for at least 60 min. Data collected during the 30 min that preceded drug administration represented basal MAP and HR.
4.3. Measurement of Plasma Norepinephrine
For the determination of plasma NE, 3 blood samples (0.2 ml each) were drawn from each rat via the arterial catheter prior to WIN55,212-2 (baseline) and 10 and 30 min after WIN55,212-2 administration. An equal volume of saline was infused after the collection of each blood sample to compensate for plasma volume loss. Blood samples were collected into heparinized tubes and centrifuged at 5000 rpm for 5 min as in our previous study (El-Mas et al., 2009). The plasma was aspirated and stored at −80°C. Plasma NE was measured by a commercially available ELISA kit (17-NORHU-E01-RES; ALPCO Diagnostics, Windham, NH) in accordance with the manufacturer’s instructions.
4.4. Immunofluorescence
Modified protocols used in previous reports (Matias et al., 2008; Wang and Abdel-Rahman, 2005) were used for TH-ir and c-Fos-ir colocalization studies. Following deep anesthesia, transcardic perfusion with 4% paraformaldehyde in phosphate buffered saline (PBS) continued for 30 min after flushing with ice-cold saline. The brain was removed, placed in the same buffer for 24 h, then transferred to 30% sucrose in PBS, pH 7.4, and kept until it sank. Serial coronal frozen brainstem sections (30 μm) containing the RVLM, rostrally from -12.8 to -11.8 mm relative to bregma (Paxinos and Watson, 2005) were cut at −24°C with a microtome cryostat (HM 505 E; Microm International GmbH, Walldorf, Germany) and collected in each well of a cell culture plate containing ice-cold PBS. Free floating sections were then washed 3X in Tris-buffered saline (TBS) for 15 min, and incubated for 3 h in blocking buffer (1% bovine serum albumin, 5% normal donkey serum in TBS containing 0.1% Tween-20%; TBST) at room temperature, before they were incubated for 48 h at 4°C in mixture of mouse monoclonal anti-tyrosine hydroxylase (TH) antibody (1:500; Chemicon., Temecula, CA) and rabbit polyclonal anti-c-Fos antibody (1:2000; Calbiochem, San Diego, CA). After 2X washes for 15 min in TBST, dual-labeling immunofluorescence was revealed by incubation for 2 h in a mixture of FITC-conjugated donkey anti-mouse and Cy3-conjugated donkey anti-rabbit (1:200; Jackson Immunoresearch, CA). Negative controls (leaving out the primary antibody) were used to establish the lack of nonspecific staining. A Zeiss LSM 510 confocal microscope was used for the visualization, acquisition, and quantification of colocalization. Images were captured by confocal laser microscopy (Carl Zeiss LSM 510, Thornwood, New York) using multi-track acquisition mode to eliminate cross talk between channels. Four to six sections per animal at the level of RVLM were examined (Paxinos and Watson, 2005). Criteria used to identify positively labeled cells were as follows: TH-ir neurons were identified by cytosolic labeling (pseudocolored green) with visible processes and a blank nuclear region (Kline et al., 2010). Fos-ir labeling was identified as nuclear staining (pseudocolored red) with a visible nucleolus. c-Fos-ir and TH-ir positive cells were counted manually using 20X objective. Cells were considered to be co-labeled if the location of nuclear Fos staining corresponded to the blank region in cytosolic labeling of TH-ir in the same focal plane as seen in the merged image. All slides were coded and the examiner was blinded to the experimental groups. If needed, for clarity, the same adjustments of the brightness and contrast of the images, obtained from the treatment and control groups, were made by Zeiss LSM Image Browser software (v.4.2) and by Adobe Photoshop (v. CS4, Adobe Systems, San Jose, CA, USA).
4.5. Drugs
WIN55,212-2, DMSO and muscimol were purchased from Sigma-Aldrich (St. Louis, MO). AM251 was purchased from Cayman Chemical (Ann Arbor, MI). Alkamus oil was purchased from Rhone-Poulenc (Cranbury, NJ). WIN55,212-2 and AM251 were dissolved in (1:1:18) mixture of DMSO/Alkamus/sterile saline. Muscimol was dissolved in sterile saline. All drugs were delivered i.c. in a volume of 5 μl/rat. Each vehicle was tested on at least three animals prior its utilization. As none of these vehicles significantly changed the basal levels of MAP and HR, we refer to both of them as vehicle.
4.6. Statistical analysis
Mean arterial pressure (MAP) was calculated as: diastolic pressure + one-third (systolic pressure - diastolic pressure). Data are expressed as mean ± S.E.M. change from their respective baseline (before injection of WIN55,212 or vehicle). BP and HR data were analyzed by repeated measures ANOVA using SPSS 16.0 statistical package for Windows®, for differences in time and treatment trends followed by a one way ANOVA to assess individual differences at different time points among different groups. Tukey’s (equal variance) and Games Howell (unequal variance) tests were used for post hoc analysis. Contrasts based on the t-test and the ANOVA error terms were used to compare pre-treatment-to-post-treatment values in each group. These contrasts examined whether there were drug-evoked changes from baseline. P < 0.05 was considered significant. A one-way ANOVA was used to evaluate the effect of various treatments on colocalization of c-Fos-ir and TH-ir in RVLM neurons (experiment 4). P<0.05 was considered significant.
Highlights.
Activation of central CB1R increased blood pressure, plasma norepinephrine level, and RVLM catecholaminergic neuronal activity and reduced heart rate.
Selective blockade of brainstem CB1R (AM251) or activation of GABAAR (muscimol) reduced WIN55,212-2-evoked neurochemical and pressor responses.
These findings suggest a pivotal role for the inhibition of GABAergic transmission in the central CB1R-evoked sympathoexcitation and pressor response in conscious rats.
Acknowledgments
Supported in part by El-Minia Faculty of Pharmacy via a scholarship provided by the Egyptian Government (Scholarship Missions Program, Ministry of Higher Education) to Badr M. Ibrahim and by NIH grant 2R01 AA07839-18.
List of non-standard abbreviations
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- BP
blood pressure
- CB1R
Cannabinoid receptor 1
- DMSO
dimethylsulfoxide
- HR
heart rate
- MAP
mean arterial pressure
- NE
norepinephrine
- RVLM
rostral ventrolateral medulla
- WIN55
212-2, (R)-(+)-[2,3-Dihydro-5-methyl-3[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate salt
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano M, Kubo T. Involvement of Both GABA-A and GABA-B Receptors in Tonic Inhibitory Control of Blood-Pressure at the Rostral Ventrolateral Medulla of the Rat. Naunyn-Schmiedebergs Archives of Pharmacology. 1993;348:146–153. doi: 10.1007/BF00164791. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clinical Pharmacology & Therapeutics. 1979;25:440–6. doi: 10.1002/cpt1979254440. [DOI] [PubMed] [Google Scholar]
- Drew GM, Mitchell VA, Vaughan CW. Glutamate spillover modulates GABAergic synaptic transmission in the rat midbrain periaqueductal grey via metabotropic glutamate receptors and endocannabinoid signaling. Journal of Neuroscience. 2008;28:808–15. doi: 10.1523/JNEUROSCI.4876-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Facilitation of Myocardial PI3K/Akt/nNOS Signaling Contributes to Ethanol-Evoked Hypotension in Female Rats. Alcoholism: Clinical and Experimental Research. 2009;33:1158–1168. doi: 10.1111/j.1530-0277.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW, Pedroso JJ, Pearlson GD. Marijuana and cocaine interactions in humans: cardiovascular consequences. Pharmacology, Biochemistry & Behavior. 1987;28:459–64. doi: 10.1016/0091-3057(87)90506-5. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiological Reviews. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Regional haemodynamic responses to the cannabinoid agonist, WIN 55212-2, in conscious, normotensive rats, and in hypertensive, transgenic rats. British Journal of Pharmacology. 2001;133:445–53. doi: 10.1038/sj.bjp.0704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennett T. Complex regional haemodynamic effects of anandamide in conscious rats. British Journal of Pharmacology. 2002;135:1889–96. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Atkinson PJ, Showalter VM, Martin BR, Abood ME. Evaluation of Cannabinoid Receptor Agonists and Antagonists Using the Guanosine-5′-O-(3-[35S]thio)-triphosphate Binding Assay in Rat Cerebellar Membranes. Journal of Pharmacology and Experimental Therapeutics. 1998;285:553–560. [PubMed] [Google Scholar]
- Hubbard JW, Buchholz RA, Keeton TK, Nathan MA. Plasma norepinephrine concentration reflects pharmacological alteration of sympathetic activity in the conscious cat. Journal of the Autonomic Nervous System. 1986;15:93–100. doi: 10.1016/0165-1838(86)90082-2. [DOI] [PubMed] [Google Scholar]
- Jelsing J, Larsen PJ, Vrang N. Identification of cannabinoid type 1 receptor expressing cocaine amphetamine-regulated transcript neurons in the rat hypothalamus and brainstem using in situ hybridization and immunohistochemistry. Neuroscience. 2008;154:641–652. doi: 10.1016/j.neuroscience.2008.03.051. [DOI] [PubMed] [Google Scholar]
- Kline DD, King TL, Austgen JR, Heesch CM, Hasser EM. Sensory afferent and hypoxia-mediated activation of nucleus tractus solitarius neurons that project to the rostral ventrolateral medulla. Neuroscience. 2010;167:510–527. doi: 10.1016/j.neuroscience.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde Inhibition of Presynaptic Calcium Influx by Endogenous Cannabinoids at Excitatory Synapses onto Purkinje Cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997;29:1204–10. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Zakrzeska A, Kurz C, Göthert M, Kwolek G, Wielgat P, Braszko J, Schlicker E. Involvement of central β2 adrenergic, NMDA and thromboxane A2 receptors in the pressor effect of anandamide in rats. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2010;381:349–360. doi: 10.1007/s00210-010-0497-6. [DOI] [PubMed] [Google Scholar]
- Matias I, Cristino L, Di Marzo V. Endocannabinoids: some like it fat (and sweet too) Journal of Neuroendocrinology. 2008;20(Suppl 1):100–9. doi: 10.1111/j.1365-2826.2008.01678.x. [DOI] [PubMed] [Google Scholar]
- Menezes RCA, Fontes MAP. Cardiovascular effects produced by activation of GABA receptors in the rostral ventrolateral medulla of conscious rats. Neuroscience. 2007;144:336–343. doi: 10.1016/j.neuroscience.2006.08.062. [DOI] [PubMed] [Google Scholar]
- Nassar N, Abdel-Rahman AA. Brainstem phosphorylated extracellular signal-regulated kinase 1/2-nitric-oxide synthase signaling mediates the adenosine A2A-dependent hypotensive action of clonidine in conscious aortic barodenervated rats. Journal of Pharmacology & Experimental Therapeutics. 2008;324:79–85. doi: 10.1124/jpet.107.129692. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. British Journal of Pharmacology. 1999;126:457–66. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Cannabinoids cause central sympathoexcitation and bradycardia in rabbits. Journal of Pharmacology & Experimental Therapeutics. 2000;294:707–13. [PubMed] [Google Scholar]
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn-Schmiedebergs Archives of Pharmacology. 2003;367:434–43. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SE, Randall MD, Gardiner SM. The in vitro and in vivo cardiovascular effects of Delta9-tetrahydrocannabinol in rats made hypertensive by chronic inhibition of nitric-oxide synthase. Journal of Pharmacology & Experimental Therapeutics. 2007;321:663–72. doi: 10.1124/jpet.106.116566. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous Cannabinoids Mediate Retrograde Signals from Depolarized Postsynaptic Neurons to Presynaptic Terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Padley JR, Li Q, Pilowsky PM, Goodchild AK. Cannabinoid receptor activation in the rostral ventrolateral medulla oblongata evokes cardiorespiratory effects in anaesthetised rats. British Journal of Pharmacology. 2003;140:384–94. doi: 10.1038/sj.bjp.0705422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam; Boston: 2005. [Google Scholar]
- Pfitzer T, Niederhoffer N, Szabo B. Central effects of the cannabinoid receptor agonist WIN55212-2 on respiratory and cardiovascular regulation in anaesthetised rats. British Journal of Pharmacology. 2004;142:943–52. doi: 10.1038/sj.bjp.0705874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. Journal of Hypertension. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Patel S, Rademacher DJ, Hopp FA, Schmeling WT, Hillard CJ. Anandamide content and interaction of endocannabinoid/GABA modulatory effects in the NTS on baroreflex-evoked sympathoinhibition. American Journal of Physiology - Heart & Circulatory Physiology. 2004;286:H992–1000. doi: 10.1152/ajpheart.00870.2003. [DOI] [PubMed] [Google Scholar]
- Showalter VM, Compton DR, Martin BR, Abood ME. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. Journal of Pharmacology and Experimental Therapeutics. 1996;278:989–999. [PubMed] [Google Scholar]
- Sidney S. Cardiovascular consequences of marijuana use. J Clin Pharmacol. 2002;42:S64–70. doi: 10.1002/j.1552-4604.2002.tb06005.x. [DOI] [PubMed] [Google Scholar]
- Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. British Journal of Pharmacology. 1996;119:107–14. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T, Bles F, Ganten D, Lang RE, Rettig R, Schwab NA. Gabaergic stimulation inhibits central actions of angiotensin II: Pressor responses, drinking and release of vasopressin. European Journal of Pharmacology. 1983;90:1–9. doi: 10.1016/0014-2999(83)90207-8. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. Br J Pharmacol. 1999;127:935–40. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Abdel-Rahman AA. Effect of chronic ethanol administration on hepatic eNOS activity and its association with caveolin-1 and calmodulin in female rats. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2005;289:G579–85. doi: 10.1152/ajpgi.00282.2004. [DOI] [PubMed] [Google Scholar]
- Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic Response to Insulin Is Mediated by Melanocortin 3/4 Receptors in the Hypothalamic Paraventricular Nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheal AJ, Bennett T, Randall MD, Gardiner SM. Cardiovascular effects of cannabinoids in conscious spontaneously hypertensive rats. British Journal of Pharmacology. 2007;152:717–24. doi: 10.1038/sj.bjp.0707410. [DOI] [PMC free article] [PubMed] [Google Scholar]