Abstract
Epicardial development is a process during which epithelial sheet movement, single cell migration and differentiation are coordinated to generate coronary arteries. Signaling cascades regulate the concurrent and complex nature of these three events. Through simple and highly reproducible assays, we identified small organic molecules that impact signaling pathways regulating these epicardial behaviors. Subsequent biochemical analyses confirmed the specificity of these reagents and revealed novel targets for the widely used Dorsomorphin (DM) and LDN-193189 molecules. Using these newly characterized reagents, we show the broad regulation of epicardial cell differentiation, sheet movement and single cell migration by Transforming Growth Factor β (TGFβ). With the DM analog, DMH1, a highly specific Bone Morphogenetic Protein (BMP) inhibitor, we demonstrate the cooperative yet exclusive role for BMP signaling in regulation of sheet migration. The action of DMH1 reveals that small organic molecules (SOM) can intervene on a single epicardial behavior while leaving other concurrent behaviors intact. All SOM data were confirmed by reciprocal experiments using growth factor addition and/or application of established non-SOM inhibitors. These compounds can be applied to cell lines or native proepicardial tissue. Taken together, these data establish the efficacy of chemical intervention for analysis of epicardial behaviors and provide novel reagents for analysis of epicardial development and repair.
Mesothelium is the simple squamous lining of the body cavities and organs (1). Mesothelia are important in development, as they are essential for blood vessel formation and organogenesis in general (2–5). Still, generation of mesothelium is complex and information on regulation of its various cell behaviors is only now emerging (6–8). The epicardium, covering the heart, is the best-studied model of mesothelial development (9, 10). During embryogenesis, the proepicardium arises independently from the heart on the sinus venosus (3, 4, 11–13). Development and differentiation of the proepicardium is dependent on multiple simultaneous cell behaviors as it migrates to the naked myocardium, adheres and spreads over the heart as an epithelial sheet (9, 14). During this lateral migration event, selected epicardial cells undergo epithelial/mesenchymal transition, migrate throughout the heart and differentiate into many lineages including vascular smooth muscle, fibroblasts, endothelial cells, and cardiomyocytes (5–7, 11, 15). Epicardial development is likely to require cooperative signaling mechanisms.
The approach to understanding complex molecular interplay controlling cell behaviors has historically used gene mutation and knockdown studies to deactivate gene products (16). While these studies have been successful, they are not without caveats as they may cause secondary effects on the system, and are expensive in terms of time, effort and materials. Additionally, knockdown or knockout studies may simultaneously remove all functions performed by the gene, including regulation by non-coding microRNAs (17, 18). An emerging alternative employs organic chemical “perturbagens” that bind to proteins, alter their function, and replace or complement gene mutation or knockdown studies (17). Small organic molecules (SOM) have an added advantage: when well-characterized, selected compounds have highly-specific activity on discrete residues of their target proteins and may affect only one of multiple functions performed by that molecule (17).
An excellent example of this concept is Dorsomorphin (DM) and its family of analogs (19, 20). These structurally-related compounds differentially target Bone Morphogenetic Protein (BMP) signaling. BMPs are part of the Transforming Growth Factorβ (TGFβ) superfamily of growth factors (21, 22) and have been implicated in regulating epicardial behaviors (23–26). TGFβ and BMP signaling occurs through stimulation of ligand-specific type-I and type II serine/threonine kinase receptors on the effector cell membrane, where they dimerize in response to ligand (27). The receptor complex stimulates signal transduction in effector cells via phosphorylation of Smad family mediator molecules, which translocate to the nucleus and stimulate transcription of target genes (27). Identification of SOMs with broad or specific inhibitory effects on these pathways would produce versatile tools to study intricate developmental processes regulated by concurrent signaling cascade mechanisms.
Chemical biology is particularly amenable for unraveling the complexity of epicardial development, where many signaling cascades coordinately impact cell behaviors. In the present study, we conducted SOM screens of epicardial differentiation, sheet movement and single cell migration to elucidate signaling pathways regulating these independent yet concurrent events. With compounds that broadly or specifically intervene on signaling pathways, we show a dependence on TGFβ signaling in the regulation of all three activities. Conversely, using a newly characterized and highly specific BMP inhibitor, the DM analog DMH1, we show that both TGFβ and BMP signal cascades interdependently regulate epicardial sheet migration. Intervention with these perturbagens demonstrates that a single epicardial behavior can be inhibited while simultaneous cell activities are left intact in both clonal cell lines and native tissue. Taken together, these data demonstrate the efficacy of chemical intervention to identify cooperative signaling in the regulation of epicardial behaviors. In addition, we provide the field with well-characterized reagents for intervention in both embryogenesis and wound healing.
Results
Specific small molecules intervene in epicardial smooth muscle differentiation
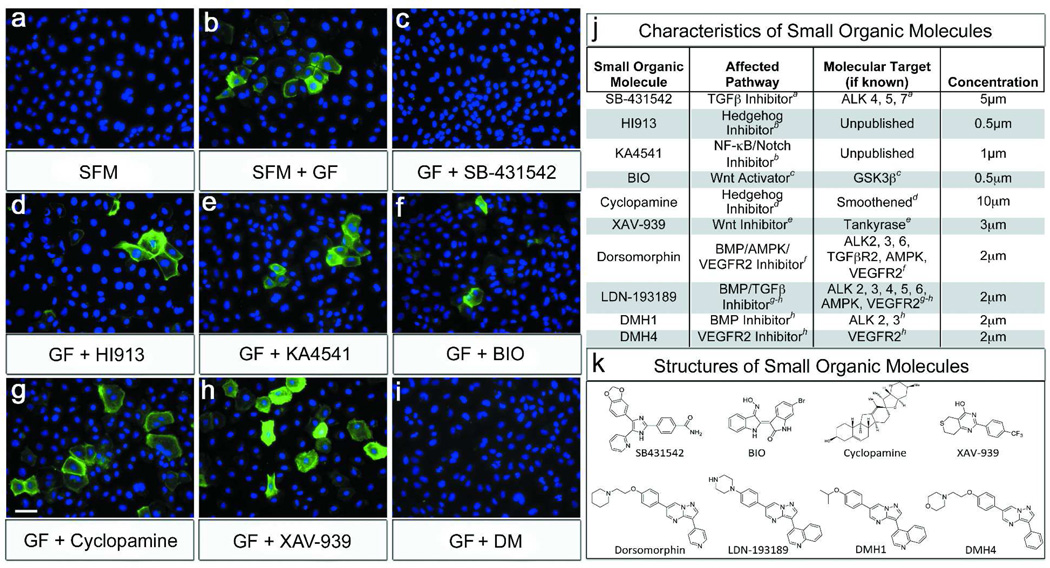
In an effort to establish a broadly applicable approach to analyze regulation of specific epicardial cell behaviors, a panel of SOMs was tested for effects on early smooth muscle differentiation. Previous work from our laboratory and others determined that expression of smooth muscle α-actin (SMA) in epicardial cells is a hallmark of epithelial/mesenchymal transition, an initial step in smooth muscle differentiation, and can be stimulated by multiple growth factors (10, 28, 29). Treatment of epicardial cell lines with a combination of known SMA-stimulating growth factors for 48 hours in serum-free medium (hereafter termed medium) evoked a robust and highly replicable SMA response (Figure 1, panel a versus b). Representative results of selected SOMs are presented in Figure 1, panels a–i. The characteristics and structures of representative molecules are listed in Figure 1, panels j–k. Most compounds had no or minimal effect on the number of cultured cells expressing SMA. One exception was the TGFβ inhibitor, SB-431542 (30), which completely ablated SMA expression (Figure 1, panel c). This is a valuable internal control, as TGFβ is known to stimulate SMA expression in epicardium. Interestingly, Dorsomorphin (DM), an inhibitor of BMP signaling cascade receptors along with other signaling pathways including vascular endothelial growth factor (VEGF) (31–33), also ablated SMA expression (Figure 1, panel i). This is an unexpected result, as BMP signaling is thought to only regulate myocardial differentiation in the heart, but is not a well-described activator of smooth muscle differentiation (27, 28, 34–36). Thus, these surprising results raised the possibility that BMP signaling may be involved in epicardial cell smooth muscle differentiation.
Figure 1.
Dorsomorphin and SB-431542 inhibit epicardial α-smooth muscle actin (SMA) expression. a–b) Epicardial/mesothelial cells (hereafter termed ‘epicardial cells’) treated with serum-free medium (hereafter termed ‘medium’) plus or minus growth factors serve as negative and positive controls for SMA expression. c) Epicardial cells stimulated with growth factors and treated with SB-431542 ablated SMA expression. d–h) Growth factor-stimulated cells treated with KA4541, BIO, Cyclopamine or XAV had no effect on SMA expression. i) Growth factor-stimulated cells treated with Dorsomorphin (DM) also strongly inhibited SMA expression. j) This information panel provides details about the SOMs tested in these studies. k) The structures of the small molecules tested in this study are presented here. [(Scale bar=50µm; Footnotes: A-(26), B-unpublished data, C-(41), D-(42), E-(43), F-(44), G-(30), H-(22) SFM=Medium; GF=Growth Factor Medium].
Application of DM analogs elucidates differential requirements for TGFβ and BMP signaling for epicardial differentiation
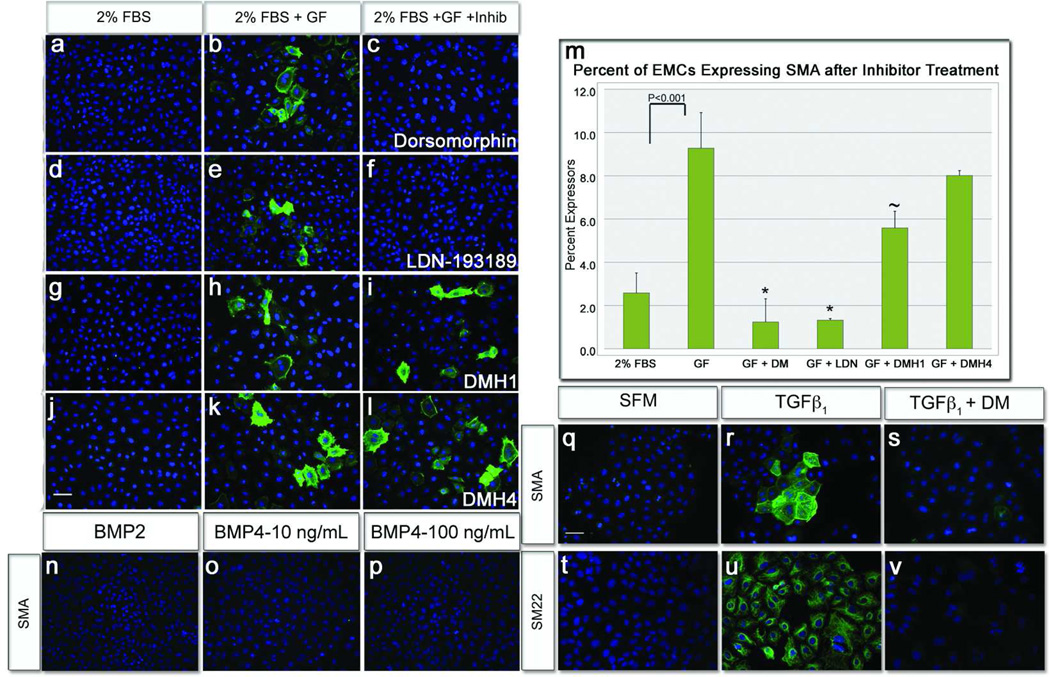
We next determined whether the previous results revealed a novel role for BMP signaling in epicardial differentiation, an effect of DM on an alternate pathway, or both. To examine these possibilities, SMA expression was assayed following treatment with 63 structural analogs of DM that have greater selectivity for individual pathways than the parent compound (33, 37). Treatment of cells with LDN-193189, an inhibitor of BMP and VEGF pathways (33, 37), eliminated SMA expression (Figure 2, panels d–f, and m). Conversely DMH1, an extremely selective inhibitor of the BMP type-I receptors Activin receptor-like kinases 2 and 3 (ALK2 and ALK3) had minimal effect (Figure 2, panels g–i, m). Additionally, DMH4, a selective inhibitor of VEGF receptor-2 (VEGFR2)(33), had no impact on epicardial SMA expression (Figure 2, panels j–m). Thus, use of these more selective inhibitors suggests that BMP signaling is not responsible for epicardial differentiation and that DM and LDN-193189 act on a previously unidentified target regulating this behavior.
Figure 2.
Analysis of growth factor regulation of SMA expression in epicardial cells. a,d,g,j) Epicardial cells cultured in low-serum medium did not express SMA. b,e,h,k) Growth factors added to low-serum medium induced high levels of epicardial SMA expression. c,f,m) Epicardial cells cultured with growth factors and treated with DM/LDN-193189 significantly inhibited SMA expression compared to untreated cells. i,m) Epicardial cells cultured with growth factors and treated with the BMP-specific inhibitor DMH1 marginally inhibited SMA expression. l,m) Epicardial cells cultured with growth factors and treated with DMH4 were unaffected (from growth factor p=0.237). n–p) Epicardial cells cultured in BMP2, or 4 did not stimulate SMA expression, at low or high concentrations. q–r, t–u) Conversely, TGFβ1 treatment stimulated expression of the early differentiation markers, SMA and SM22. s,v) TGFβ1–induced SMA and SM22 expression was ablated by addition of DM. [n=≥20 images; Scale bar=50µm; (*p<0.01; ~p<0.05) TGFβ=Transforming Growth Factor β; BMP=Bone Morphogenetic Protein].
To explore these unanticipated results, we conducted the inverse experiment adding specific growth factors to epicardial cultures and determining their effects on SMA expression. Addition of BMP2 and 4, both canonical stimulators of the BMP receptors ALK2/3 (38, 39) and highly expressed in epicardial progenitors (23, 34), had no effect on SMA or SM22 expression at multiple concentrations (Figure 2, panels n–p, Supplemental Figure S1). TGFβ1 was the only growth factor to induce expression of these early smooth muscle differentiation markers in epicardial cells (Figure 2, panels q–r, t–u), reflecting results obtained from other groups (8, 27, 28). Interestingly and consistent with our previous data, this stimulation was completely ablated by addition of DM, identical to the inhibitory effects of SB-431542 (Figure 2, panels s, v). Corroborating our previous results in which the BMP inhibitor, DMH1, had little or no effect on TGFβ stimulation of epicardial differentiation (Figure 2, panel i). These results support the data presented above, that BMP has no effect on this epicardial differentiation and that DM and LDN-193189 may affect this particular behavior in epicardial cells via inhibition of TGFβ signaling.
Identification of novel DM analog targets reveals differential regulation of epicardial receptors
To precisely determine the signal cascades inhibited by DM and its analogs, in vitro kinase inhibitory activities (as assessed by IC50, concentration causing 50% inhibition) against potential BMP and TGFβ receptors were performed. As expected, all DM analogs tested strongly inhibited ALK1 and ALK2, both type-I receptors selective for BMP ligands (Table 1;(38, 39)). Our previous work has shown that DM analogs also potently inhibited ALK3, another BMP type-I receptor (33). Importantly, DMH1 had minimal activity on any of the TGFβ receptors tested (Table 1: ALK4, ALK5 and TGFβR2; (33)). Conversely and unexpectedly, our data revealed for the first time that the widely-used DM and LDN-193189 inhibited TGFβR2, a type-II receptor for TGFβ (40).
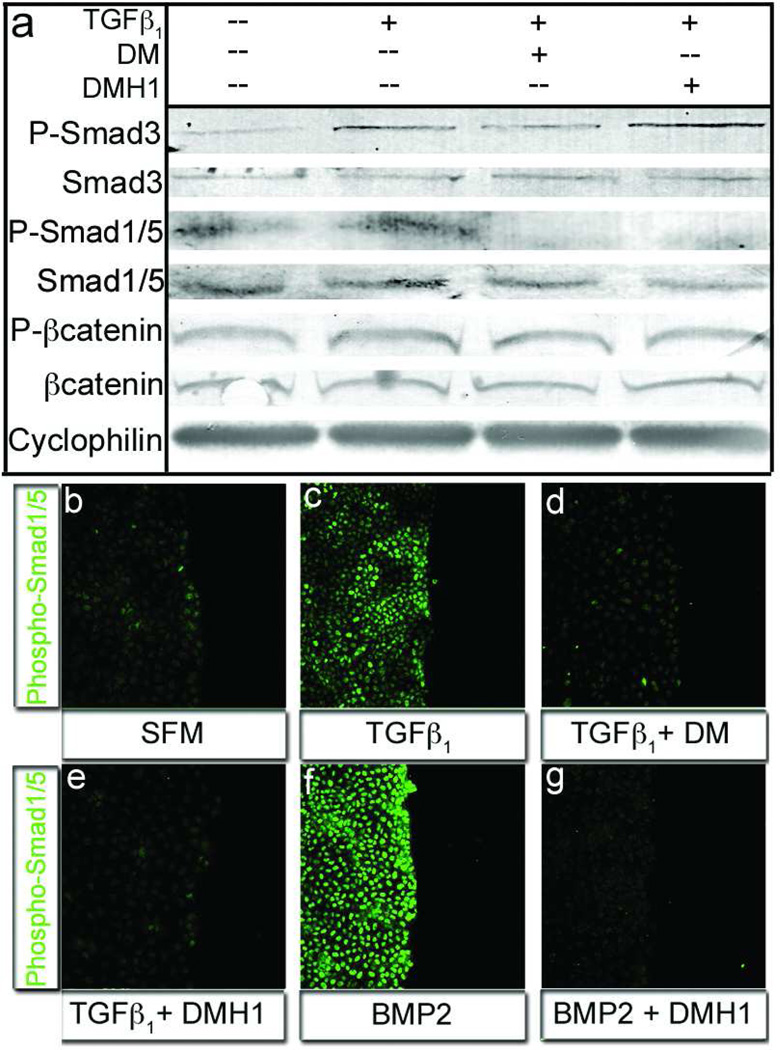
We next examined whether the apparent differential effects of DM and DMH1 on BMP and TGFβ receptors in biochemical analyses extended to epicardial cells. Since BMP and TGFβ signaling occur via distinct receptors to trigger Smad1/5/8 and Smad2/3 phosphorylation, respectively (21, 22), we utilized pathway-specific Smad phosphorylation to determine the inhibitory precision of DM and DMH1 in epicardial cells. TGFβ1 administration induced Smad3 phosphorylation, and consistent with our in vitro kinase assay data, DM, but not DMH1, inhibited TGFβ1-dependent Smad3 phosphorylation (Figure 3, panel a). Conversely, both DM and DMH1 ablated Smad1/5 phosphorylation below baseline (Figure 3, panel a). Total Smad3, Smad1/5, and housekeeping gene (cyclophilin) protein levels were unchanged in all conditions. Additionally, the Wnt signaling cascade was unaffected under all conditions, further conferring specificity to the DM analogs (Figure 3, panel a). Immunofluorescence analysis demonstrated that BMP2 stimulated high levels of phospho-Smad1/5 nuclear localization, which was completely inhibited by both DM and DMH1 (Figure 3 f–g and data not shown). TGF-β had minor stimulatory effect on nuclear localization, which was also inhibited by DM and DMH1 (Figure 3, panel c–e). Collectively, our data confirm that TGFβ, but not BMP, signaling is critical for epicardial cell differentiation and identify new targets of the widely used DM and LDN-193189 molecules. With a precise understanding of the activities of the DM analogs in epicardial cells it was now possible to examine the concurrent regulation of additional behaviors by the two signaling pathways.
Figure 3.
DMH1, but not Dorsomorphin, specifically inhibits Smad1/5 activity. a) The TGFβ responsive Smad3 molecule was activated in the presence of TGFβ1 and inhibited with addition of Dorsomorphin, but not DMH1 (row: P-Smad3). Alternatively the phosphorylation of the BMP-responsive Smad1/5 molecules was lost when cells were treated with DM or DMH1 (row: P-Smad1/5). Lysates from epicardial cells in all treatments expressed equal amounts of Cyclophilin (loading control), Smad1/5, and β-Catenin total proteins (rows: Cyclophilin, Smad3, Smad1/5, and βCatenin, respectively). β-Catenin phosphorylation was unchanged, conferring specific activity to DM and DMH1 on Smad transduction molecules (row: P-βCatenin). b–c) Phospho-Smad1/5 nuclear localization was minimal in serum free medium and was marginally upregulated by TGFβ1. f) Alternatively, BMP2 treatment greatly increased activated Smad1/5 nuclear localization. e,g) TGFβ-stimulated cells wounded, treated with DM and DMH1 did not localize Smad1/5 to the nucleus. [n=3 P-Smad3=Phosphorylated Smad3, P-Smad1/5=Phosphorylated Smad1/5 Protein, P-βCatenin=Phosphorylated βCatenin.]
Elimination of BMP signaling inhibits epicardial sheet movement, but not differentiation
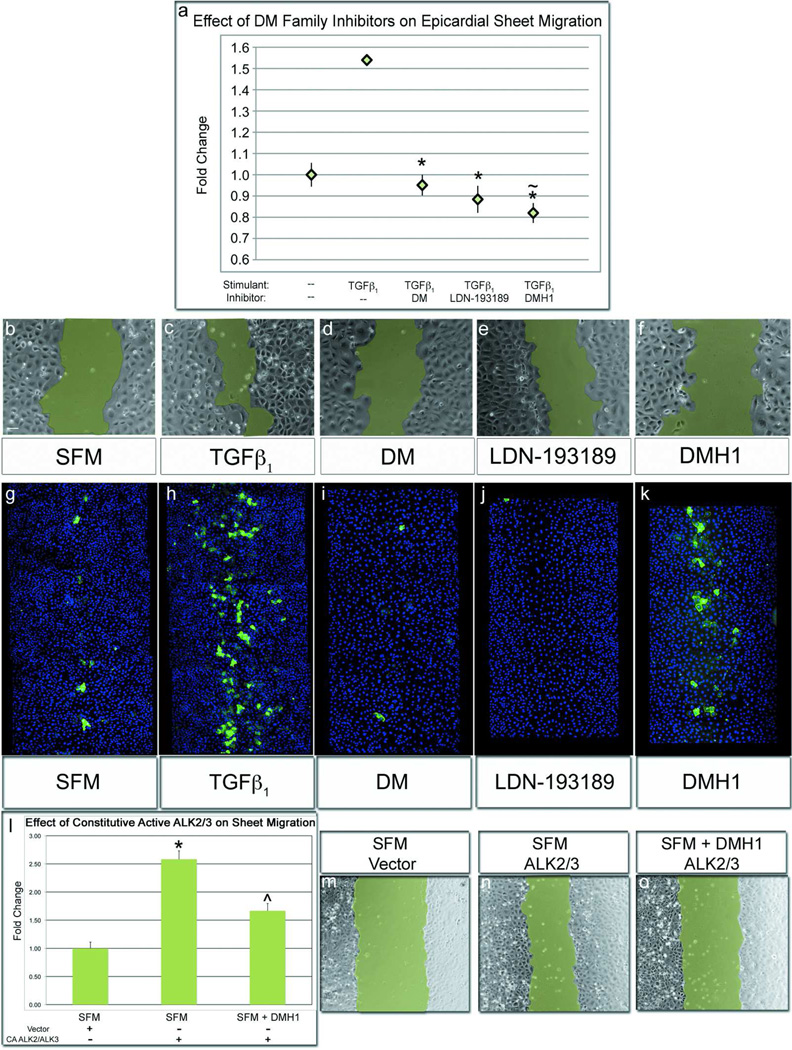
A more precise understanding of DM and DMH1 activities at the level of BMP and TGFβ receptors enabled us to use these reagents to explore whether TGFβ and/or BMP signaling regulate epicardial cell behaviors other than SMA expression. Epithelial sheet migration is a function basic to epicardial cells and can be analyzed after scratch injury (3, 4, 6). In the presence of TGFβ1, epicardial wounds incubated for six hours exhibited significantly increased regrowth rate (Figure 4, panels a–c). Application of DM and LDN-193189 inhibited sheet migration to control rate, indicating that elimination of TGFβ and/or BMP signaling disrupts this function (Figure 4, panel d–e). Interestingly, DMH1, the exquisitely selective BMP inhibitor, intervened in TGFβ-induced sheet migration; having the strongest inhibitory effect of all DM analogs (Figure 4, panel f compared to a). In fact, DMH1 significantly decreased migration rate below the negative control, similar to the strong TGFβ inhibitor SB-431542 (Supplementary Figure S2, panels a–c). Because of this surprising result, we determined whether activated BMP receptors might overcome the requirement for TGFβ1 ligand. Thus, we overexpressed constitutively active ALK2/3 and assayed sheet movement in the absence of TGFβ1. Overexpression of these receptors strongly enhanced epicardial sheet migration, which was again inhibited upon application of DMH1 (Figure 4, panels l–o). Further in subsequent experiments, we will demonstrate that exogenous application of BMP ligands can also stimulate sheet movement in the absence of TGFβ (Figure 7, panel f–g). Thus, activated BMP signaling in the absence of TGFβ is sufficient to elicit this response, complementing the strong loss-of-migration phenotype observed with DMH1. Because addition of TGFβ is the only exogenously applied growth factor in this context, it is possible that an endogenous source of BMPs exists or that the BMP targets ALK1–3, may be directly activated by TGFβ1 ligand. We perform experiments outlined below which resolve this question (Figure 7). Taken together, these data illustrate the concurrent requirement for TGFβ and BMP signaling in the regulation of epicardial sheet movement.
Figure 4.
DMH1 strongly inhibits epicardial sheet movement, but not SMA expression. Epicardial sheet migration rate was assessed by wound healing assays with representative images of wounds at six hours in b–f. a,c) Wounding epicardial sheets in the presence of TGFβ1 significantly increased sheet migration rate in comparison cells wounded in medium. a,d–e) Epicardial wounds cultured with TGFβ1 and concurrently treated with DM/LDN-193189 healed at medium-equivalent rates. a,f) Interestingly, sheets wounded with TGFβ1-and the BMP inhibitor, DMH1, healed at a rate significantly slower than unstimulated sheets. g) Epicardial sheets wounded in medium spontaneously expressed SMA after an 18-hour incubation. h) When wounds were cultured with TGFβ1, SMA expression was strongly increased. i–j) Conversesly, when sheets cultured with TGFβ1 and treated with DM/LDN-193189, SMA expression was inhibited. e) DMH1 marginally inhibited TGFβ1-stimulated SMA expression. l, n) Epicardial sheet migration was strongly stimulated by constitutively active ALK2/3 l,o) Migration was modestly inhibited by treatment of constitutively active-ALK2/3 cells with DMH1, which differs from the significant inhibition that DMH1 exerts on sheet migration in wildtype cells. [n≥8 wounds; Scale bar=50µm; (*p<0.001 from TGFβ1 stimulated cells; ~p<0.001 from untreated cells; ^~p<0.001 from untreated CA-ALK2/3); CA-ALK2/3=Constitutively Active-ALK2/3].
Figure 7.
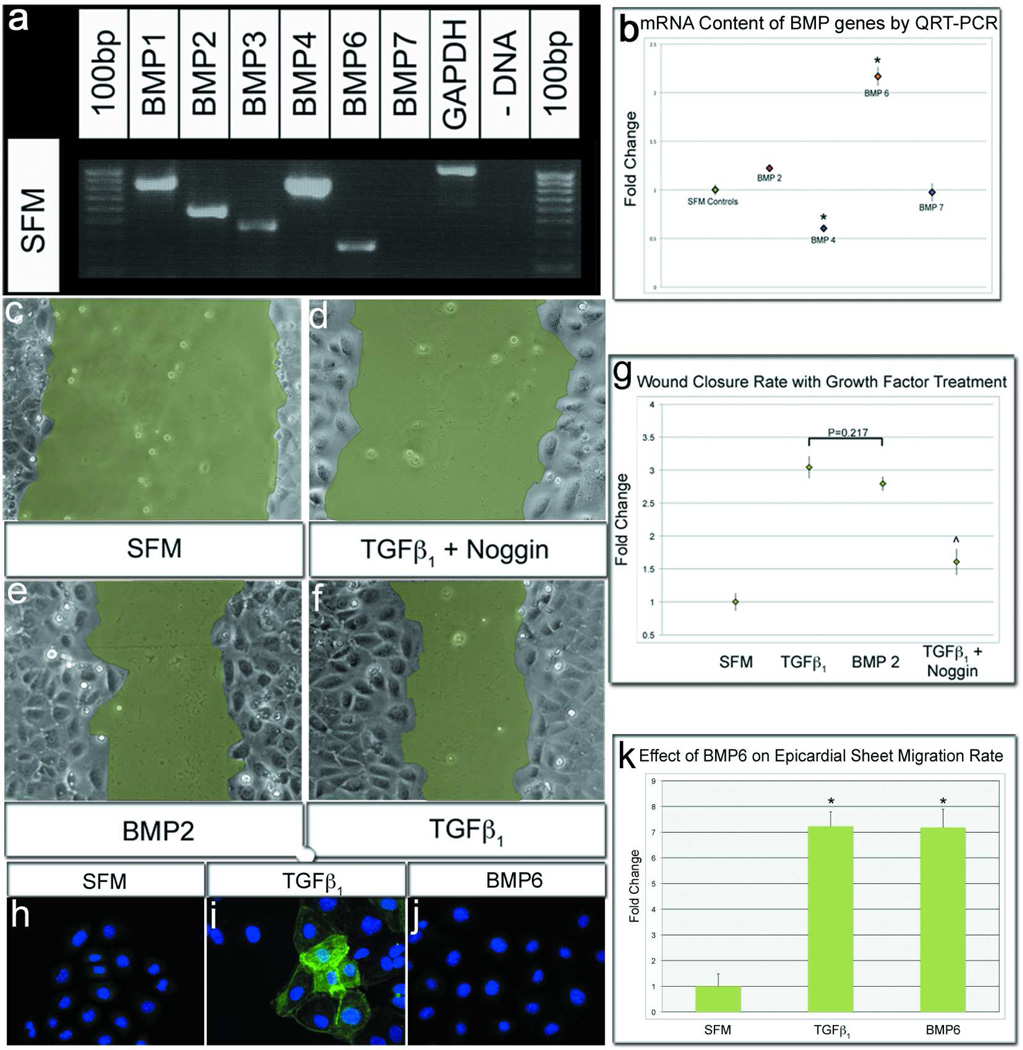
Epicardial cells express BMP factors that regulate epicardial behaviors. a) Epicardial cells express a multitude of BMP mRNAs when assayed by RT-PCR. b) Only BMP6 expression level was significantly increased after TGFβ1 treatment when assayed by QRT-PCR. c,d,f,g) Epicardial cells treated with TGFβ1 and Noggin (200ng/mL) inhibited epicardial sheet migration significantly when compared to untreated, TGFβ1-stimulated epicardial sheets. e) Epicardial cells treated with BMP2, only, stimulated sheet migration with a rate equivalent to TGFβ1 stimulated sheets. f–h) Similar to BMP2, Epicardial cells stimulated with BMP6 in medium did not express SMA when compared to TGFβ1 stimulated cells. However, BMP6 treatment enhanced sheet migration to a rate equivalent to TGFβ1 stimulated sheets. [(p=0.92). n=40 images; Scale bar=50µm; *p≤0.001; ^p≤0.001 from TGFβ1 treated].
Simultaneous with migration of a wounded epithelial sheet is the expression of SMA in cells on its leading edge (41). Analysis of SMA expression during sheet migration afforded us the opportunity to examine two distinct epicardial cell behaviors at the same time, and to explore whether specific perturbagens reveal differential regulation of these events. Sheets incubated with TGFβ1 exhibited a marked increase in SMA expression, along with robust sheet migration (Figure 4, panel h). Application of DM, LDN-193189 or SB-431542, all shown to inhibit TGFβ/Smad2/3 signaling in this system, strongly blocked both behaviors (Figure 4, panels i–j, Supplemental Figure S2, panel b). Interestingly, while DMH1 had the greatest inhibitory effect on sheet migration, SMA expression was untouched (Figure 4, panel k). These data demonstrate that inhibition of TGFβ has broad inhibitory effects on epicardial behaviors, while BMP disruption is highly selective for sheet migration. Application of DM and DMH1 to epicardial cells allowed the simultaneous analysis of multiple cell behaviors, and revealed specific intervention in only one behavior while leaving the other concurrent behaviors intact.
DMH1 does not ablate epicardial single cell migration
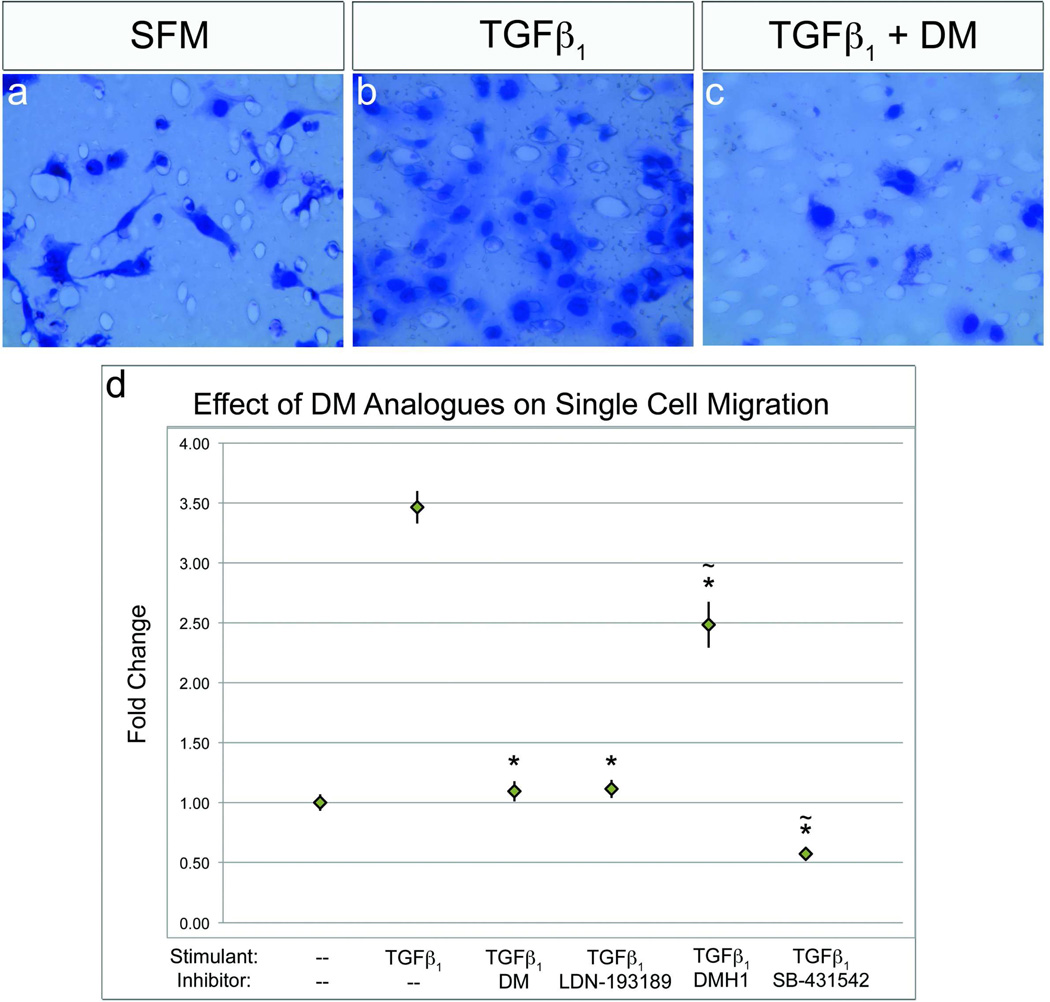
Our current data suggest that BMP signaling regulates sheet movement, only, in epicardial cells. However, single cell migration is also a critical behavior for epicardially-derived cells (4) and, as such, we analyzed the impact of BMP signaling on this activity. It has been previously noted that TGFβ stimulates single cell invasive migration (42), thus we used this factor as an activator of the behavior and perturbed the behavior with the DM analogs. When epicardial cells were stimulated with TGFβ1 a significant increase in single cell migration rate occurred compared to controls (Figure 5, panels a versus b and d). Interestingly, application of DMH1 did not interfere with single cell migration (Figure 5, panel d). Conversely, DM, LDN-193189 and SB-431542 all strongly inhibited single cell migration, (Figure 5, panels c and d). BMP2 and 6 were tested for their influence on single cell migration in the same assay and, as expected, did not stimulate this behavior, corroborating the DMH1 data (Supplemental Figure S3, panels b–d). Taken together, these data indicate that BMP signaling specifically regulates epicardial sheet migration, having little influence on other behaviors in epicardial cell lines.
Figure 5.
DMH1 does not ablate single cell migration. a) Epicardial cells cultured in medium have minimal single cell migration in Boyden chamber assays. b,d) Single cell migration is significantly enhanced by stimulation with TGFβ1. c,d) TGFβ1 stimulation was inhibited by DM/LDN-193189 to a migration rate equivalent with medium, (p=0.056). d) TGFβ1 stimulation was only marginally inhibited by DMH1, unlike the TGFβ inhibitor SB-431542, which ablated this behavior. [n=30 images; (*p<0.001 from TGFβ1 ~p<0.001 from SFM)].
DMH1 inhibits sheet migration, but not early differentiation, in proepicardial explants
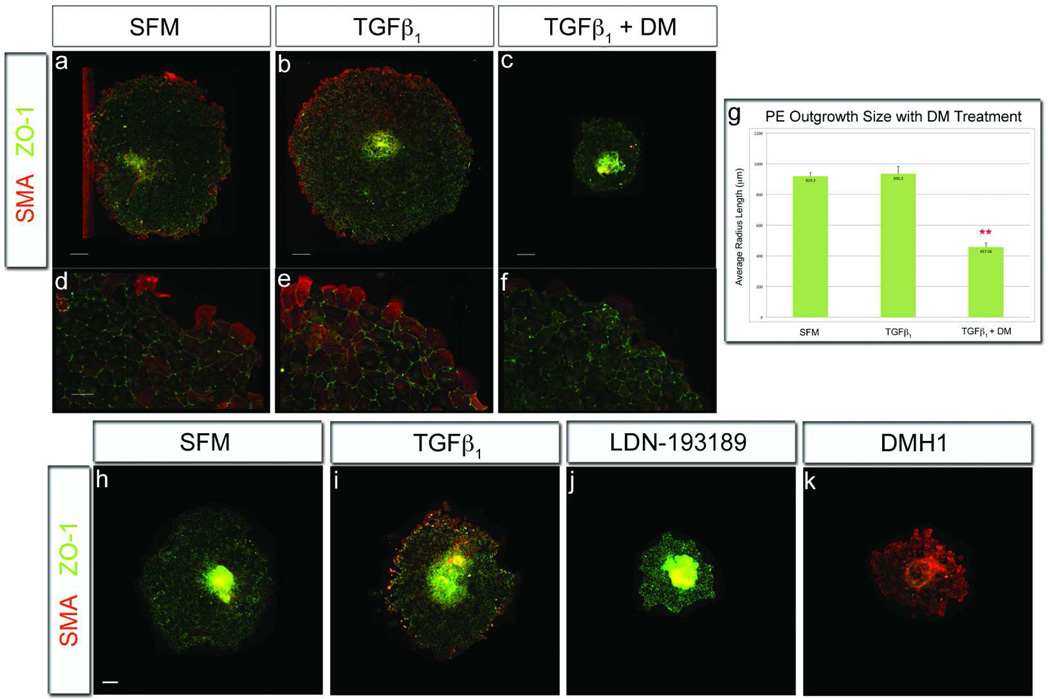
The proepicardium is the source of epicardial cells in the developing embryo (3, 4, 12, 13). Several groups have shown that proepicardial explants grown in vitro retain the developmental properties analyzed in this study (4, 6, 28, 43). Thus, it was essential to determine whether SOMs are effective tools to modulate specific cell behaviors in native embryonic epicardial tissues. Proepicardial explants grown in basal medium spread on fibronectin to form a radial outgrowth with the average radius of 904 µm (Figure 6, panel a, d, g). These outgrowths had membrane localized Zonula Occludins-1 (ZO-1; a commonly used epithelial junction marker (44)), and SMA expressing cells at their periphery. Proepicardial explants incubated in TGFβ1 had outgrowths of approximately the same size as untreated explants (avg. radius=1,003 µm; Figure 6, panel g). This similarity was unsurprising given the native mobility of embryonic epicardial tissue. However, TGFβ1 evoked increased peripheral SMA expression and stimulated expression within the epicardial sheet itself (Figure 6, panel b, e). In contrast, when proepicardial explants were grown in the presence of DM, there was a significant decrease in the spread of the epicardial sheet at 24 hours (avg. radius=489 µm; Fig. 6, panel c, f, g). Similar results occurred with LDN-1923189 treatment (Figure 6, panel j). Additionally, in DM and LDN-193189 treated explants, SMA expression was restricted to the peripheral cells and was greatly diminished in intensity compared to TGFβ1-treated cultures. In contrast, treatment of proepicardial explants with DMH1 resulted in the greatest inhibition of epithelial sheet migration. At the same time, DMH1 treated outgrowths displayed the highest level of SMA expression, with abundant numbers of positive cells at the periphery and within the sheet itself (Figure 6, panel k). These data corroborate our previous studies using adult epicardial cell lines and suggest that BMP signaling is required for epicardial sheet migration while having minimal, or even perhaps a permissive effect on SMA expression. The specific inhibition of sheet migration from both clonal epicardial cells and native embryonic tissue further demonstrates the efficacy of DMH1 in perturbation of a single behavior while leaving other functions unaffected.
Figure 6.
DMH1 inhibits sheet migration, but not SMA expression, in proepicardial explants. HH stage 16 proepicardia were excised from chick embryos and incubated for 24 hours. a,d) Proepicardia incubated in medium exhibited a uniformly spread epicardial cell sheet with SMA expression at the distal edge and ZO-1, marking epithelial cells, in the interior of the sheet. b,e,g) Proepicardia cultured in TGFβ1 had upregulated SMA expression centrally and peripherally in the outgrowths, but TGFβ1 treatment did not increase outgrowth migration rate from medium controls. c,f,g,j) Proepicaria stimulated with TGFβ1 and treated by DM/LDN-193189 had reduced epicardial sheet migration and SMA expression. k) Proepicardia stimulated by TGFβ1 and treated with DMH1 inhibited outgrowth migration, but had enhanced SMA expression and diminished zonula occludins-1 protein expression throughout the epicardial sheet. [Measurements assessed per condition=40; Scale bars=200µm for A–C and 50µm for D–F; (*p<0.001; n≥5 proepicardia); ZO-1=Zonula Occludins-1].
BMPs are expressed by epicardial cells and regulate cellular behaviors
The present study suggests that TGFβ and BMP signaling cascades have both interdependent and independent roles governing epicardial development. While TGFβ and BMP signaling could display some cross-talk amongst transduction molecules after receptor activation, the most parsimonious explanation of this result is that BMPs are made by epicardial cells, and work together with exogenously applied TGFβ to regulate sheet migration. Indeed, previous work has shown that both epicardium and compact myocardium produce TGFβ ligands and BMPs (6, 36, 45). To determine whether epicardial cells express BMPs, cultures were incubated in medium for 24 hours and assessed by RT-PCR. As seen in Figure 7, panel a, a myriad of BMPs were detected under basal conditions. In subsequent studies to quantify BMP expression using QRT-PCR, TGFβ1 treatment enhanced expression of BMP6 only (Figure 7, panel b).
Finally, although epicardial cells make BMP growth factors, it is possible, due to effector cross-talk between TGFβ ligand and BMP signal receptors, that BMP ligands are not required to stimulate sheet migration. Previous studies indicate that while TGFβ and BMP-induced signaling cascades are often independent, TGFβ may activate BMP receptors (22, 28). To test whether BMP itself is a stimulating ligand, the classic BMP extracellular sequestering molecule, Noggin (46), was used to remove any endogenously produced BMP ligands. Wounded epicardial sheets stimulated by TGFβ1 and treated with Noggin had a significant reduction in sheet migration when compared to TGFβ1 treatment alone (Figure 7, panel d, g). This result was similar to, but not as pronounced as, the reduction observed with DMH1 treatment (Compare Figure 7, panel g with Figure 4, panel a). Thus, Noggin and DMH1 both inhibit extracellular stimulation of BMP receptors, but DMH1 has greater efficacy and specificity for sheet migration. In a complementary experiment, application of the canonical BMP pathway-activating protein, BMP2, enhanced epicardial sheet migration rate beyond that observed with endogenous levels of BMP (Figure 7, panels e, g). This differs from the effect of BMP2 on SMA expression, where addition of the growth factor had no stimulatory effect (Figure 2, panel n). Finally, as TGFβ1 treatment enhanced BMP6 expression, this molecule was tested for effects on SMA expression and sheet migration. As observed with BMP2 treatment, BMP6 had no effect on smooth muscle differentiation (Figure 7, panels h–j), but strongly enhanced sheet migration (Figure 7, panel k), suggesting that BMP2 and 6 have similar stimulatory functions in this system. Interestingly as shown above, BMP2 and 6 have no effect on single cell migration (Supplemental Figure S3, panels b–d). Taken together, these data indicate that extracellular BMP growth factors are required to stimulate epicardial sheet migration in conjunction with TGFβ.
Conclusion
Here, we present simple, highly reproducible screens to identify SOM perturbagens of epicardial behaviors. Further, we have characterized selected compounds to precisely detail their molecular specificity and have identified important new targets of the widely used DM and LDN-193189 molecules. With these reagents, it was possible to determine their potential intervention in three concurrent epicardial behaviors. Specifically, we demonstrate the broad requirement for TGFβ in epicardial differentiation, sheet movement and single cell migration, while determining that BMP signaling is essential for only sheet movement, with little to no impact on the two other functions. Together, these data illustrate that well-characterized analog families of SOMs are valuable tools to intervene on specific behaviors in multi-faceted systems. Additionally, we are the first to show cooperative regulation of epicardial sheet migration by the TGFβ and BMP signaling cascades. Using the DM analog family permitted a co-analysis of multiple signaling factor contributions to this epicardial behavior. During development, cells respond concurrently to a myriad of signals. It is highly probable that multiple signal cascades can and do contribute to the regulation of a single behavior. Developing tools like the DM analog family will permit the identification of cooperative regulation of epicardial development, which might have been overlooked using less sensitive perturbation techniques.
Methods
Quantification
For the assays described below statistics and percent closure were performed in Microsoft Excel. Error bars represent the standard error of the mean; student t-tests were used to determine significance. All images being compared were adjusted equally.
Cell culture, cell and tissue processing, antibodies and immunofluorescence analysis
Epicardial cell culture conditions and processing were standard (10) as was slide preparation (47). Commercially available antibodies and their working dilutions are given in supplemental information.
SMA expression and wound healing assays
Initially, 105 epicardial cells were seeded to 4-well glass chamber slides (NUNC Lab-Tek 154526) in standard epicardial cell medium (10). After the 18 hour incubation, the medium was changed to 2% FBS; 2% FBS/DMEM, supplemented with TGFβ1, PDGF and EGF (Millipore GF111 & GF149, BD Biosciences 354001, respectively) at 10ng mL−1 each, to induce epicardial cell differentiation with simultaneous addition of SOMs at the concentrations given in Figure 1, panel j. For induction by TGFβ1 alone, medium was supplemented with 20ng mL−1 TGFβ1 in 2µM DMSO. The negative control consisted of medium with DMSO. For induction by BMP2, 4 and 6 (R&D Systems 355-BM, 314-BM-010, 6325-BM, respectively) growth factor was added to SFM. Noggin (R&D Systems 3344-NG) was added at 200ng mL−1 to the TGFβ1-supplemented medium. In all situations, cells were incubated for 48 hours, until being processed.
To assay early differentiation and sheet migration rate, 2.5×105 cells were seeded to glass 4-well chamber slides and incubated overnight in standard epicardial cell media. After 18 hours incubation, cells were equilibrated in mediums. After six-hours incubation, confluent cell sheets were scratched with a 200µL pipette tip, incubated for an additional 18 hours and then analyzed for SMA expression as above. Sheet movement was quantified at zero and six hours after wounding. For ALK2/3 overexpression assays, 5µg of CA-ALK2/3 plasmids were expressed with the Nucleofector II Device (Amaxa Biosystems) using Solution L, program A-020. 2.5×105 cells were added to each well of 4-well slide and after 48 hours incubation sheets were wounded and analyzed. This work was conducted at the Vanderbilt CISR.
Smad Phosphorylation Assays
For analysis by western blot, cells were grown to confluence, serum starved and stimulated with TGFβ as above. Lysates were collected, total protein measured, and immunoblotted as previously reported (47) AP detection of immunoreactive bands was standard (47). For analysis by IF, cells were seeded, treated and injured as above for western blot analysis. Cells were processed for Phospho-Smad1/5 antibody as per Cell Signaling (9516). Z-series were captured at VUMC CISR and two 0.6µm Z-planes were projected with LSM Image Browser.
IC50 Determination
Kinase binding and confirmatory in vitro kinase assays were performed by KinomeScan at www.kinomescan.com.
RNA collection, cDNA synthesis and Quantitative RT-PCR
18 hours preceding experimental intervention, 2×105 cells were seeded in triplicate to a 6-well plastic culture dish (Falcon 353502). Next, cells were stimulated with the TGFβ1-induction conditions described above. After 24 hours incubation, cells were lysed (Qiashredder, Qiagen 79654), and RNA was purified as described (48). Specific primers with a melting temperature of 59°C were designed from the Rattus norvegicus cDNA database using PrimerBlast (NCBI) and are listed in Supplementary Table S4. QRT-PCR was performed on the 7900HT Fast Real-Time PCR System (ABI) (using the VUMC DNA resources core). Gene expression was compared using TaqMan Gene Expression assays: BMP2-Rn01484736_M1, BMP4-Rn00432087_m1, BMP6-Rn00432095_m1, GAPDH-Rn99999916_s1, 18s-HS99999901_s1. Data was managed in RQ Manager 1.2 software. All statistics were performed on the ΔCT values.
Modified Boyden Chamber Migration Assay
3.0×105 cells per experiment were pelleted for 10 minutes at 1500 RPM in a desktop microcentrifuge and resuspended in 400uL of control and experimental media described above. The cells were seeded to the basket of an 8.0µM cell culture insert (Millicell P18P01250) and placed in 600µL of the same media in a 24-well plastic culture dish (Falcon). Cells were incubated for 4 hours, and processed with standard methods (48). Fifteen phase images were captured on an Olympus BX60 microscope and Olympus Camera.
Chicken Proepicardium Explant Differentiation and Migration Assay
Gallus gallus 16.5 Proepicaria were dissected from embryos and placed on 4-well glass chamber slides coated with 5ug (cm2)−1 Fibronectin (Sigma F4759). Proepicardia were incubated for 24 hours in TGFβ1-induced culture conditions (above) and processed for immunochemistry with standard methods (43). Images were captured and processed at VUMC EBC. Proepicardial explant size was ascertained with phase-contrast imaging and by total-protein content measurement (Supplementary Figure S5).
Supplementary Material
Acknowledgement
We gratefully acknowledge J. Roland of the VUMC EBC, and VUMC CISR for help in image acquisition, VUMC DNA Resources Core for QRT PCR. We received intellectual contributions from: J. Hao, P. Miller, and N.Winters. These studies were supported by NIH grants 5T32 HL007411, DK 83234-01. CCH was supported by the Cali Family Fund for FOP Research, Vanderbilt Institute for Clinical and Translational Research, Department of Veterans Affairs CDTA, VA Merit, NIH/NHLBI R01HL104040, and U01HL100398.
Footnotes
Author contributions: EEC, DMB, RTT, CCH designed the research; EEC, MM, RTT, CRH performed the research; EEC, DMB analyzed the data and wrote the paper.
Supporting Information Available: This material is available free of charge via the Internet.
Literature Cited
- 1.Wilm B, Ipenberg A, Hastie N, Burch J, Bader D. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 2.Mikawa T, Fischman DA. Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci USA. 1992;89:9504–9508. doi: 10.1073/pnas.89.20.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circulation Research. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 4.Reese DE, Mikawa T, Bader DM. Development of the Coronary Vessel System. Circulation Research. 2002;91:761–768. doi: 10.1161/01.res.0000038961.53759.3c. [DOI] [PubMed] [Google Scholar]
- 5.Dettman R, Denetclaw W, Ordahl C, Bristow J. Common epicardial origin of coronary vascular smooth muscle, perivascular fibroblasts, and intermyocardial fibroblasts in the avian heart. Developmental Biology. 1998;193:169–181. doi: 10.1006/dbio.1997.8801. [DOI] [PubMed] [Google Scholar]
- 6.Ishii Y, Garriock RJ, Navetta AM, Coughlin LE, Mikawa T. BMP signals promote proepicardial protrusion necessary for recruitment of coronary vessel and epicardial progenitors to the heart. Developmental Cell. 2010;19:307–316. doi: 10.1016/j.devcel.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellgren AM, Smith CL, Olsen GS, Eskiocak B, Zhou B, Kazi MN, Ruiz FR, Pu WT, Tallquist MD. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circulation Research. 2008;103:1393–1401. doi: 10.1161/CIRCRESAHA.108.176768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austin AF, Compton LA, Love JD, Brown CB, Barnett JV. Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFβ. Dev. Dyn. 2008;237:366–376. doi: 10.1002/dvdy.21421. [DOI] [PubMed] [Google Scholar]
- 9.Männer J, Pérez-Pomares JM, Macías D, Muñoz-Chápuli R. The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs. 2001;169:89–103. doi: 10.1159/000047867. [DOI] [PubMed] [Google Scholar]
- 10.Wada AM, Smith TK, Osler ME, Reese DE, Bader DM. Epicardial/Mesothelial Cell Line Retains Vasculogenic Potential of Embryonic Epicardium. Circulation Research. 2003;92:525–531. doi: 10.1161/01.RES.0000060484.11032.0B. [DOI] [PubMed] [Google Scholar]
- 11.Cai C-L, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikawa T, Borisov A, Brown AM, Fischman DA. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus: I. Formation of the ventricular myocardium. Dev Dyn. 1992;193:11–23. doi: 10.1002/aja.1001930104. [DOI] [PubMed] [Google Scholar]
- 13.Nahirney PC, Mikawa T, Fischman DA. Evidence for an extracellular matrix bridge guiding proepicardial cell migration to the myocardium of chick embryos. Dev Dyn. 2003;227:511–523. doi: 10.1002/dvdy.10335. [DOI] [PubMed] [Google Scholar]
- 14.Komiyama M, Ito K, Shimada Y. Origin and development of the epicardium in the mouse embryo. Anatomy and embryology. 1987;176:183–189. doi: 10.1007/BF00310051. [DOI] [PubMed] [Google Scholar]
- 15.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Developmental Biology. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 16.Hood L, Heath JR, Phelps ME, Lin B. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- 17.Stockwell BR. Exploring biology with small organic molecules. Nature. 2004;432:846–854. doi: 10.1038/nature03196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poliseno L, Salmena L, Zhang J, Carver B, Haveman WJ, Pandolfi PP. A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature. 2010;465:1033–1038. doi: 10.1038/nature09144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao J, Daleo M, Murphy C, Yu P, Ho J, Hu J, Peterson R, Hatzopoulos A, Hong C. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE. 2008:e2904. doi: 10.1371/journal.pone.0002904. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao J, Ho J, Lewis J, Karim K, Daniels R, Gentry P, Hopkins C, Lindsley C, Hong C. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010:245–253. doi: 10.1021/cb9002865. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Wang X-F. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii Y, Garriock R, Navetta A, Coughlin L, Mikawa T. BMP signals promote proepicardial protrusion necessary for recruitment of coronary vessel and epicardial progenitors to the heart. Developmental Cell. 2010:307–316. doi: 10.1016/j.devcel.2010.07.017. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Wijk B, van den Berg G, Abu-Issa R, Barnett P, van der Velden S, Schmidt M, Ruijter J, Kirby M, Moorman A, van den Hoff M. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circulation Research. 2009:431–441. doi: 10.1161/CIRCRESAHA.109.203083. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin n, Compton L, Love J, Sanchez N, Brown C, Barnett J. Migration and differentiation of epicardial cells stimulated by TGFβ1, TGFβ2, or BMP-2 do not require the Type III Transforming Growth Factor β receptor. FASEB J. 2010 In. [Google Scholar]
- 26.Schlueter J, Männer J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Developmental Biology. 2006:546–558. doi: 10.1016/j.ydbio.2006.03.036. In. [DOI] [PubMed] [Google Scholar]
- 27.Giehl K, A M. Moving on: Molecular mechanisms in TGFbeta-induced epithelial cell migration. Signal Transduction. 2006;6:355–364. [Google Scholar]
- 28.Olivey HE, Mundell NA, Austin AF, Barnett JV. Transforming growth factor-β stimulates epithelial–mesenchymal transformation in the proepicardium. Dev. Dyn. 2005;235:50–59. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mellgren A, Smith C, Olsen G, Eskiocak B, Zhou B, Kazi M, Ruiz F, Pu W, Tallquist M. Platelet-derived growth factor receptor beta signaling is required for efficient epicardial cell migration and development of two distinct coronary vascular smooth muscle cell populations. Circulation Research. 2008:1393–1401. doi: 10.1161/CIRCRESAHA.108.176768. In. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Molecular Pharmacology. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 31.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS ONE. 2008;3:e2904. doi: 10.1371/journal.pone.0002904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlueter J, Männer J, Brand T. BMP is an important regulator of proepicardial identity in the chick embryo. Developmental Biology. 2006;295:546–558. doi: 10.1016/j.ydbio.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Svensson EC. Look Who's Talking: FGFs and BMPs in the Proepicardium. Circulation Research. 2009;105:406–407. doi: 10.1161/CIRCRESAHA.109.205203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Wijk B, van den Berg G, Abu-Issa R, Barnett P, van der Velden S, Schmidt M, Ruijter JM, Kirby ML, Moorman AFM, van den Hoff MJB. Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circulation Research. 2009;105:431–441. doi: 10.1161/CIRCRESAHA.109.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuny GD, Yu PB, Laha JK, Xing X, Liu J-F, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell D, Pobre EG, Mulivor AW, Grinberg AV, Castonguay R, Monnell TE, Solban N, Ucran JA, Pearsall RS, Underwood KW, Seehra J, Kumar R. ALK1-Fc inhibits multiple mediators of angiogenesis and suppresses tumor growth. Molecular cancer therapeutics. 2010;9:379–388. doi: 10.1158/1535-7163.MCT-09-0650. [DOI] [PubMed] [Google Scholar]
- 39.Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009:343–355. doi: 10.1016/j.cytogfr.2009.10.007. In. [DOI] [PubMed] [Google Scholar]
- 40.Giehl K, Menke A. Moving on: Molecular mechanisms in TGFbeta-induced epithelial cell migration. Signal Transduction. 2006:355–364. In. [Google Scholar]
- 41.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- 42.Dokic D, Dettman R. VCAM-1 inhibits TGFbeta stimulated epithelial-mesenchymal transformation by modulating Rho activity and stabilizing intercellular adhesion in epicardial mesothelial cells. Developmental Biology. 2009:489–504. doi: 10.1016/j.ydbio.2006.08.054. In. [DOI] [PubMed] [Google Scholar]
- 43.Pennisi D, Mikawa T. FGFR-1 is required by epicardium-derived cells for myocardial invasion and correct coronary vascular lineage differentiation. Developmental Biology. 2009;328:148–159. doi: 10.1016/j.ydbio.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–L534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- 45.Olivey HE, Svensson EC. Epicardial-myocardial signaling directing coronary vasculogenesis. Circulation Research. 2010;106:818–832. doi: 10.1161/CIRCRESAHA.109.209197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 47.Smith TK, Bader DM. Characterization of Bves expression during mouse development using newly generated immunoreagents. Dev. Dyn. 2006;235:1701–1708. doi: 10.1002/dvdy.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development. Developmental Biology. 2008;317:282–295. doi: 10.1016/j.ydbio.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.