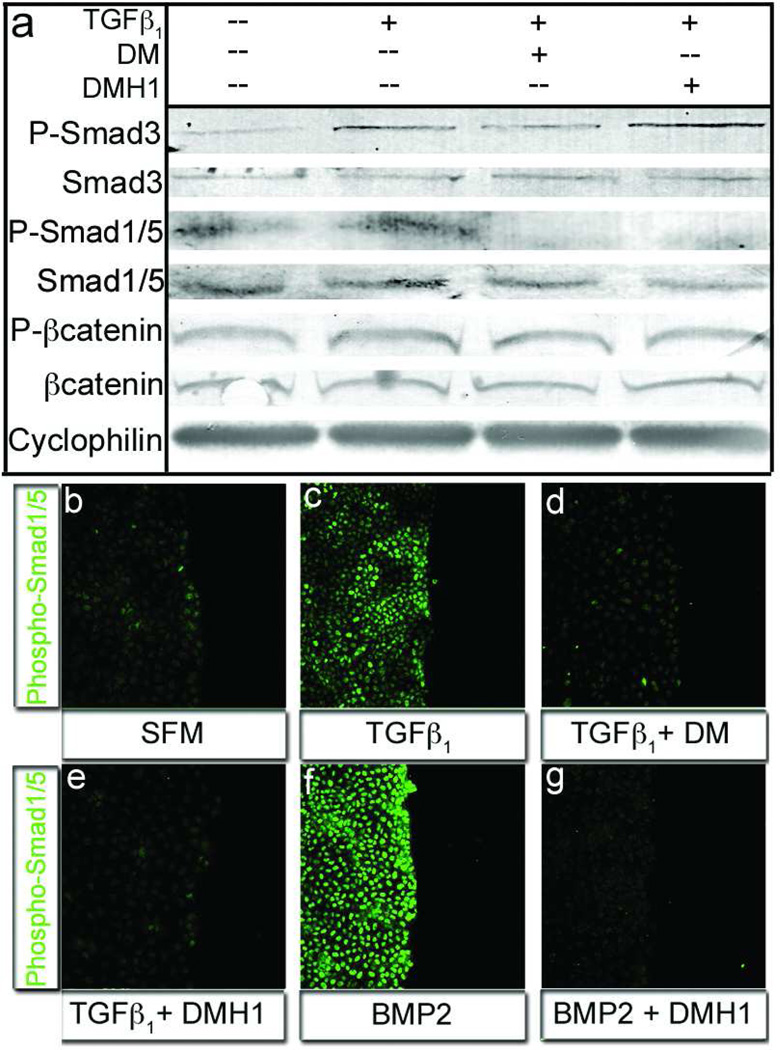
Figure 3.
DMH1, but not Dorsomorphin, specifically inhibits Smad1/5 activity. a) The TGFβ responsive Smad3 molecule was activated in the presence of TGFβ1 and inhibited with addition of Dorsomorphin, but not DMH1 (row: P-Smad3). Alternatively the phosphorylation of the BMP-responsive Smad1/5 molecules was lost when cells were treated with DM or DMH1 (row: P-Smad1/5). Lysates from epicardial cells in all treatments expressed equal amounts of Cyclophilin (loading control), Smad1/5, and β-Catenin total proteins (rows: Cyclophilin, Smad3, Smad1/5, and βCatenin, respectively). β-Catenin phosphorylation was unchanged, conferring specific activity to DM and DMH1 on Smad transduction molecules (row: P-βCatenin). b–c) Phospho-Smad1/5 nuclear localization was minimal in serum free medium and was marginally upregulated by TGFβ1. f) Alternatively, BMP2 treatment greatly increased activated Smad1/5 nuclear localization. e,g) TGFβ-stimulated cells wounded, treated with DM and DMH1 did not localize Smad1/5 to the nucleus. [n=3 P-Smad3=Phosphorylated Smad3, P-Smad1/5=Phosphorylated Smad1/5 Protein, P-βCatenin=Phosphorylated βCatenin.]