Figure 3.
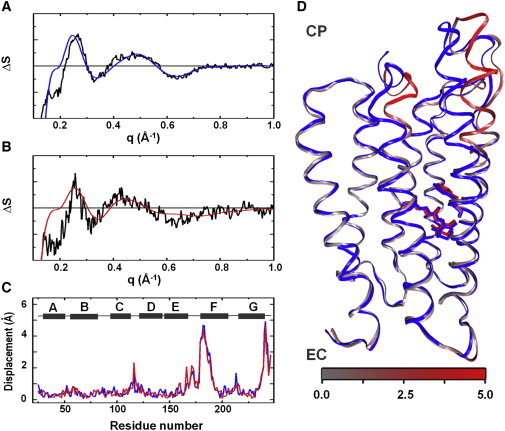
Structural modeling of WAXS data. Overlay between the basis spectrum from SVD (black) and the optimal theoretical difference WAXS curve (blue, red) from structural refinement for (A) native pR and (B) 13-I-pR. (C) Displacement plot for Cα-atoms between the resting state and the transient conformation as a function residue number for the optimal models of native pR (blue) and 13-I-pR (red). Helix locations (gray) are represented as a function of residue number (above). (D) Overlay of the resting state homology model (blue) and the refined transient conformation model of native pR. The transient conformation is colored gray to red depending upon the amplitude of the movement on Cα atoms. The all-trans and 13-cis retinal conformations and the Trp197 are represented as sticks. EC, Extracellular side; CP, cytoplasmic side.
