Figure 4.
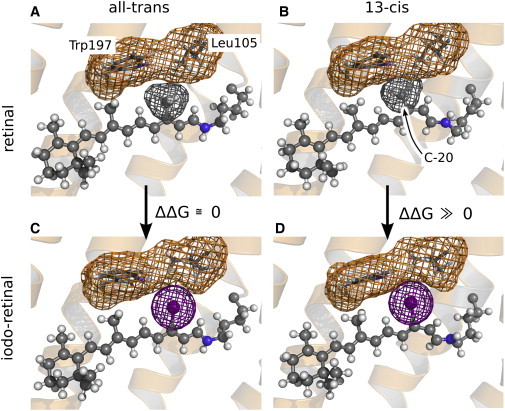
Native retinal (A and B) and iodoretinal (C and D), either in the all-trans (A and C) or in the 13-cis state (B and D). The retinal is shown in ball-and-sticks representation, and the side chains of residues Leu105 and Trp197 as sticks. The van der Waals surface of the C-20 methyl group, the iodine, and of Leu105/Trp197 is indicated (gray, purple, or orange mesh, respectively). (B and D) In 13-cis, replacing C-20 by iodine induces a steric clash with Leu105 and Trp197, leading to an unfavorable increased free energy of the protein conformation. (A and C) In all-trans, that replacement has little effect on the free-energy of the conformation. For ease of understanding, we refer to the 13-I-pR retinal conformation as all-trans and 13-cis in analogy to that used for describing native pR, although (strictly speaking) the opposite nomenclature should be applied for the 13-I-pR retinal.
