Abstract
Recent work has demonstrated the impact of dysfunction of the GABAergic signaling system in brain and the resultant behavioral pathologies in subjects with autism. In animal models, altered expression of Fragile X mental retardation protein (FMRP) has been linked to downregulation of GABA receptors. Interestingly, the autistic phenotype is also observed in individuals with Fragile X syndrome. This study was undertaken to test previous theories relating abnormalities in levels of FMRP to GABAA receptor underexpression. We observed a significant reduction in levels of FMRP in the vermis of adults with autism. Additionally, we found that levels of metabotropic glutamate receptor 5 (mGluR5) protein were significantly increased in vermis of children with autism vs. age and postmortem interval (PMI) matched controls. There was also a significant decrease in level of GABAA receptor beta 3 (GABRβ3) protein in vermis of adult subjects with autism. Finally, we found significant increases in glial fibrillary acidic protein (GFAP) in vermis of both children and adults with autism when compared with controls. Taken together, our results provide further evidence that altered FMRP expression and increased mGluR5 protein production potentially leads to altered expression of GABAA receptors.
Keywords: FMRP, mGluR5, GABRβ3, autism, vermis
Introduction
Autism is a neurodevelopmental disorder first systematically described by Leo Kanner based purely on behavioral observations (Kanner, 1943; Realmuto and Azeem, 2008). It is characterized by ritualized or stereotyped behavior, deficits in communication, and abnormalities in social interaction (APA, 1994). Recent evidence indicates an increased incidence of autism of unknown origin (Fombonne et al., 2006). A resurgence of interest in identifying the biologic underpinnings of this debilitating disease has been helped by the availability of a well characterized set of postmortem brains belonging to subjects with autism, matched against a cohort of normal control brains, resulting in a number of important, well replicated findings (Blatt et al., 2001, Fatemi and Halt 2001; Perry et al., 2001; Purcell et al., 2001; Fatemi et al., 2002a, 2005, 2009a,b, 2010; Araghi-Niknam and Fatemi, 2003; Palmen et al., 2004; Laurence and Fatemi, 2005; Yip et al., 2007; Oblak et al., 2009).
One of the important recent findings in autism deals with the abnormality of GABAergic neurotransmission. Our laboratory has shown a number of salient findings describing a major deficit with the GABAergic system in autism over the past decade including: significant downregulation of glutamic acid decarboxylase 65 and 67 kDa (GAD65 and GAD67) proteins, the rate limiting enzymes that convert glutamate to GABA (Fatemi et al., 2002a); reduction in blood and brain levels of Reelin glycoprotein (Fatemi et al., 2002b, 2005); and global reductions in GABAA and GABAB receptors in three brain areas in autism (Fatemi et al., 2009a,b, 2010). These abnormalities have now been replicated by other laboratories showing the validity of these observations (Blatt, 2005; Yip et al., 2007; Garbett et al., 2008; Oblak et al., 2009).
The autistic phenotype is also seen to occur in a number of other disorders including tuberous sclerosis, Asperger syndrome, and specifically, Fragile X syndrome (FXS). FXS is one of the most common causes of inherited mental retardation with a prevalence of 1:4,000 in males and 1:8,000 in females (Crawford et al., 1999; Oostra and Willemsen, 2009). FXS is caused primarily by expansion of a CGG repeat in the 5’ untranslated region of the FMR1 gene, which is located on the X chromosome (Oostra and Willemsen, 2009). Indeed, FXS may be considered the first example of a trinucleotide repeat expansion mutation (Oostra and Willemsen, 2009).
The Fragile X mental retardation protein (FMRP) is an RNA-binding protein (De Rubeis and Bagni, 2010). FMRP shuttles between the nucleus and cytoplasm and is involved in regulation of multiple steps in post-transcriptional events such as splicing, nuclear export, stability, and localization and translation of various RNAs (Keene, 2007; De Rubeis and Bagni, 2010). Any major abnormality in function of FMRP may profoundly affect control of multiple downstream genes leading to a group of pathologies. For example, expansion of the trinucleotide repeat in the 5’ untranslated region of the FMR1 gene leads to transcriptional silencing of this gene and loss of FMRP expression leading to Fragile X syndrome which is well characterized by mental retardation and autistic behavior. A variant form of abnormalities in FMRP expression is also associated with Fragile X associated tremor-ataxia syndrome (FXTAS), which is characterized by progressive ataxia and intention tremor (De Rubeis and Bagni, 2010). FMRP is highly expressed in the brain and mainly localized to the cytoplasm and, at lower levels, to the nucleus of the neuron (Feng et al., 1997). Additionally, FMRP can be observed not only in neuronal soma but localized along the dendrites and the base of synaptic spines and in axonal growth cones as well as in mature axons (Antar et al., 2004; Centonze et al., 2008; De Rubeis and Bagni, 2010). During the passage of FMRP from neuronal soma to synapse, it partners with a number of proteins, various mRNAs and even non-coding RNAs giving rise to a large complex known as the messenger ribonucleoprotein particle (mRNP) which upon reaching the synapse is translationally unrepressed and released via neuronal stimulation leading to change in spine morphology and ultimately affecting synaptic plasticity (Dölen and Bear, 2008).
Several recent intriguing publications have reported on decreased expression of multiple GABAA receptor mRNA species (α1, α3, α4, β1, β2, γ1, γ2, δ) in Fragile X mice, a validated model for Fragile X mental retardation syndrome (El Idrissi et al., 2005; D’Hulst et al., 2006; Gantois et al., 2006). Additionally, D’Hulst et al., (2006) also found significant reduction in three GABAA receptors in Fragile X Drosophila melanogaster. These authors posited that the global underexpression of GABAA receptors in these animal models could be an evolutionarily conserved hallmark of Fragile X syndrome (D’Hulst et al., 2006).
Previous reports have shown presence of autistic behavior in 15-33% of patients with FXS (Cohen, 1995; Bailey et al., 2000; Kauffman et al., 2004; Hatton et al., 2006). Indeed, Hatton et al., (2006) suggested that lower levels of FMRP expression may be the contributory cause of autistic behavior and intellectual deficits in children with Fragile X syndrome (Hatton et al., 2006). By the same token, Gothelf et al., (2008) recently reported a correlation between lower levels of FMRP, aberrant behavior with various abnormalities in brain size (increased size of caudate nucleus and decreased size of posterior cerebellar vermis, amygdala and superior temporal gyrus) in a group of FXS patients. We therefore decided to investigate for the first time whether FMRP levels could be lower in the vermis of children and adults with autism as compared to age and postmortem interval (PMI) matched control subjects. This study was also undertaken to buttress the previous theories relating abnormalities in levels of FMRP to GABAA receptor underexpression observed by us and others in brains from subjects with autism.
Materials and Methods
Tissue Preparation
All experimental procedures were approved by the Institutional Review Board of the University of Minnesota School of Medicine. Postmortem blocks of vermis were obtained from the Autism Research Foundation and various brain banks (NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD; TARF; the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program). The tissue samples (Table 1) were prepared as described previously (Fatemi et al., 2009a,b, 2010).
Table 1.
Demographic Data for Subjects with Autism and Controls
| Case | Dx | Sex | Age | PMI (Hrs.) | Ethnicity | Medication History | Cause of Death | Seizure | MR |
|---|---|---|---|---|---|---|---|---|---|
| 4670 | Control | M | 4 | 17 | Caucasian | None | Commotio Cordis | No | No |
| 4898 | Control | M | 7 | 12 | Caucasian | Concerta, Clonidone | Drowning | No | No |
| 1674 | Control | M | 8 | 36 | Caucasian | None | Drowning | No | No |
| 4787 | Control | M | 12 | 15 | African American | Singulair, Albuteral, Prednisone, Claritin | Asthma | No | No |
| 1823 | Control | M | 15 | 18 | Caucasian | None | MVA | No | No |
| 6396 | Control | M | 18 | 19.83 | Unknown | None | Unknown | No | No |
| 1846 | Control | F | 20 | 9 | Caucasian | None | MVA | No | No |
| 6316 | Control | F | 32 | 28.92 | Unknown | None | Unknown | No | No |
| 1169 | Control | M | 33 | 27 | African American | Metoclopramide, Claritin D | Dilated Cardiomyopathy (morbid obesity) | No | No |
| 1376 | Control | M | 37 | 12 | African American | None | ASCVD | No | No |
| 6420 | Control | M | 41 | 30.4 | Unknown | None | Heart Attack | No | No |
| 7002 | Autism | F | 5 | 32.73 | Asian | None | Drowning | No | No |
| 1349 | Autism | M | 5 | 39 | Caucasian | None | Drowning | No | No |
| 5666 | Autism | M | 8 | 22.16 | Caucasian | None | Cancer | Yes | No |
| 4231 | Autism | M | 8 | 12 | African American | Zyprexa, Reminyl | Drowning | No | Yes |
| 5342 | Autism | F | 11 | 12.88 | Caucasian | Adderall, Dexedrine, Dilantin, Klonopin, Lamictal, Tegretol, Topomax | Drowning | Yes | Yes |
| 4899 | Autism | M | 14 | 9 | Caucasian | None | Drowning | No | No |
| 6294 | Autism | M | 16 | 24 | Asian | Allegra, Buspirone, Topamax | Seizure disorder | No | No |
| 6337 | Autism | M | 22 | 25 | Caucasian | Abilify, Lamictal, Zonegran | Aspiration | Yes | No |
| 6994 | Autism | M | 29 | 43.25 | Caucasian | Allegra, Geodon, Tegretol | Seizure (suspected) | Yes | No |
| 6640 | Autism | F | 29 | 17.83 | Caucasian | Luvox | Seizure disorder | Yes | No |
| 6677 | Autism | M | 30 | 16.06 | Caucasian | None | Heart Failure (congestive) | No | No |
| 5173 | Autism | M | 30 | 20.33 | Caucasian | Cisapride, Clorazepate, Depakote, Dilantin, Folic Acid, Mysoline, Phenobarbital, Prilosec, Propulsid, Reglan, Tranxene | Gastrointestinal bleeding | Yes | No |
| 5027 | Autism | M | 37 | 26 | African American | None | Obstruction of bowel due to adhesion | No | No |
| 6401 | Autism | M | 39 | 13.95 | Caucasian | None | Cardiac Tamonade | No | No |
| 6469 | Autism | F | 49 | 16.33 | Caucasian | Coumadin, Effexor, Erythromycin, Prevacid, Risperidal, Metformin, Neurontin, Propranolol, Synthroid | Pulmonary Arrest | No | Yes |
| 4498 | Autism | M | 56 | 19.48 | Caucasian | Cogentin, Haldol, Lithobid, Thorazine, Xanax | Anoxic encephalopathy | Yes | No |
Dx, diagnosis; Hrs, hours; PMI, postmortem interval; M, male; F, female; EtOH, alcohol; MVA, motor vehicle accident; MR, Mental retardation
SDS-PAGE and Western Blotting
Tissue samples from vermis (N=11 control, N=16 autistic), were prepared and subjected to SDS PAGE and western blotting as described previously (Fatemi et al., 2009a,b, 2010). The primary antibodies used were: anti-fragile X mental retardation protein (FMRP) (ab17722, Abcam (Cambridge, MA), 1:500), anti-metabotropic glutamate receptor 5 (mGluR5) (ab53090-100, Abcam Inc. (Cambridge, MA), 1:300), anti-GABAA receptor beta 3 (GABRβ3) (NB300-199, Novus Biologicals (Littleton, CO), 1:1,000), anti-glial fibrillary acidic protein (GFAP) (G3893, Sigma-Aldrich (St. Louis, MO), 1:2,000), anti-neuronal specific enolase (NSE) (ab16808, Cambridge, MA), 1:2,000), and anti-β actin (A5441, Sigma Aldrich (St. Louis, MO), 1:5,000). Secondary antibodies used were A9169 (Sigma Aldrich, (St. Louis, MO) goat anti-rabbit IgG 1:80,000) and A9044 (Sigma Aldrich, (St. Louis, MO) rabbit anti-mouse IgG 1:80,000). The molecular weights of approximately 224 kDa (dimer) and 112 kDa (monomer) for mGluR5; 73 kDa (FMRP); 52 kDa (GABRβ3). We have observed a range of molecular weights for GABRB3 including 52 kDa and 56 kDa which agrees with the expected molecular weight of 51-56 kDa according to the antibody data sheet from Novus Biologicals, Inc. and from previously published results (Bureau and Olsen, 1990; Sarto et al., 2002; Samaco et al., 2005); 50, 46, 42 and 38 kDa (GFAP; all bands measured together), 46 kDa (NSE), and 42 kDa (β-actin) immunoreactive bands were quantified with background subtraction. As many as 2–5 bands can be seen in some preparations of FMRP (private communication with Dr. J. Darnell of Rockefeller University; Darnell et al., 2009) however, in our preparations we can distinctly see up to two clear bands generally, which were measured together. Results obtained were based on at least two independent experiments.
Statistical Analysis
Statistical analysis of protein data was performed as previously described (Fatemi et al., 2009a,b). We investigated the potential confounding effects of age, postmortem interval, and gender by examining group differences with postmortem interval as covariates. We further analyzed data based on stratification by age groups, i.e. adults (above age 18) and children (ages 18 and under). Analysis of medication and presence of seizures as confounds could not be tested when data were further stratified by age. While the impact of these factors could not be accurately verified, previous work by our group showed lack of such effects on GABAA receptor abnormalities in cerebellar tissues from a different group of subjects with autism (Fatemi et al., 2009a,b).
Results
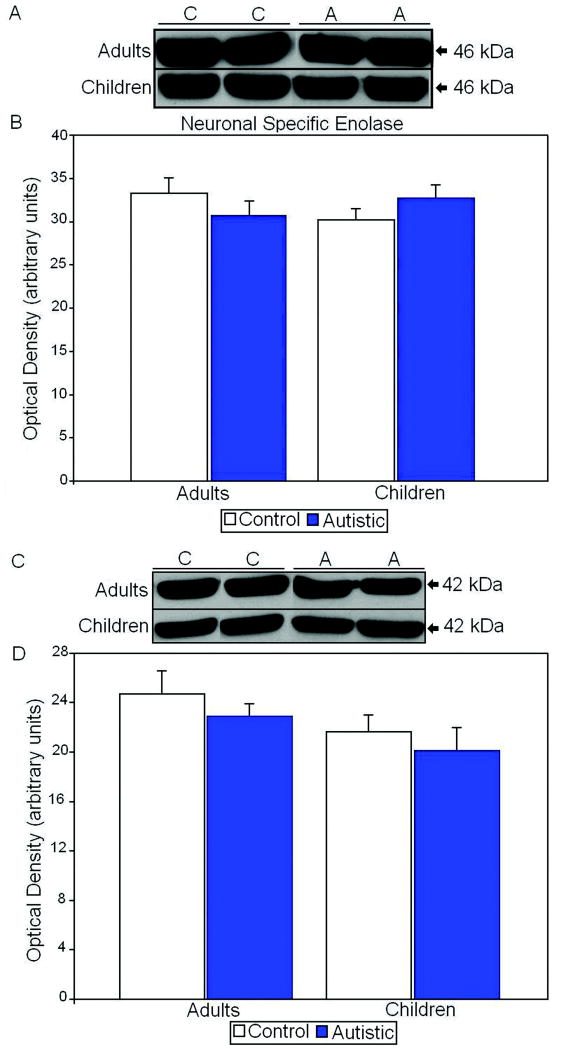
All proteins of interest were normalized against both neuronal specific enolase (NSE) and β-actin and are shown as ratios of the various proteins to NSE and β-actin. We chose NSE as a housekeeping gene to account for any changes in neuronal number between subjects with autism and controls. In adults and children there were no significant differences in levels of NSE between subjects with autism and matched controls (Figure 1; Table 2). β-actin was also used as a housekeeping gene as to account for any differences in neurons and glia. Similar to NSE there were no significant differences in adults and children when comparing subjects with autism and matched controls (Figure 1; Table 3). These results indicate that any changes in proteins of interest are not due to overall differences in neuronal cell number.
Figure 1.
A. Representative samples of neuronal specific enolase from control (C) and autistic (A) subjects. B. Mean NSE values for control and autistic (filled histogram bars) subjects are shown for vermis. C. Representative samples of β-actin from control and autistic subjects. D. Mean β-actin values for autistic (filled histogram bars) and control subjects are shown for vermis. (Error bars expressed as standard error of the mean.)
Table 2.
Western Blotting Results for FMRP, mGluR5, GABRβ3, and NSE in Vermis
| Adults | Control | Autistic | Change | P |
|---|---|---|---|---|
| FMRP/ NSE | 0.048 ± 0.025 | 0.012 ± 0.016 | ↓75% | 0.028 |
| mGluR5 Dimer/ NSE | 0.110 ± 0.112 | 0.077 ± 0.108 | ↓30% | ns |
| mGluR5 Total/ NSE | 0.139 ± 0.115 | 0.100 ± 0.115 | ↓28% | ns |
| mGluR5Dimer/mGluR5Total | 0.945 ± 0.064 | 0.603 ± 0.327 | ↓36% | ns |
| GABRβ3 /NSE | 0.057 ± 0.019 | 0.036 ± 0.008 | ↓37% | 0.031 |
| NSE | 33.3 ± 3.8 | 30.7 ± 5.28 | ↓7.8% | ns |
| Age ± SD (years) | 32.6 ± 7.89 | 35.7 ± 10.9 | ↑9.5% | ns |
| PMI ± SD (years) | 21.5 ± 10.1 | 22.0 ± 8.91 | ↑2.3% | ns |
| Gender | 3M:2F | 7M:2F | -- | -- |
|
| ||||
| Children | Control | Autistic | Change | P |
|
| ||||
| FMRP/ NSE | 0.308 ± 0.132 | 0.206 ± 0.191 | ↓33% | ns |
| mGluR5 Dimer/ NSE | 0.065 ± 0.041 | 0.198 ± 0.044 | ↑204% | 0.0023 |
| mGluR5 Total/ NSE | 0.066 ± 0.044 | 0.204 ± 0.046 | ↑209% | 0.0042 |
| mGluR5Dimer/mGluR5Total | 0.897 ± 0.058 | 0.972 ± 0.017 | ↑8.4% | 0.049 |
| GABRβ3 /NSE | 0.038 ± 0.015 | 0.043 ± 0.021 | ↑13% | ns |
| NSE | 30.2 ± 3.44 | 32.7 ± 4.04 | ↑8.2% | ns |
| Age ± SD (years) | 10.7 ± 5.28 | 9.57 ± 4.28 | ↓11% | ns |
| PMI ± SD (years) | 19.6 ± 8.45 | 21.7 ± 11.3 | ↑11% | ns |
| Gender | 6M:0F | 5M:2F | -- | -- |
ns, not significant
Table 3.
Western Blotting Results for FMRP, mGluR5, GABRβ3, GFAP, and β-Actin in Vermis
| Adults | Control | Autistic | Change | P |
|---|---|---|---|---|
| FMRP/ β-actin | 0.064 ± 0.031 | 0.016 ± 0.026 | ↓75% | 0.038 |
| mGluR5 Dimer/ β-actin | 0.145 ± 0.144 | 0.092 ± 0.126 | ↓36% | ns |
| mGluR5 Total/ β-actin | 0.183 ± 0.147 | 0.123 ± 0.138 | ↓33% | ns |
| mGluR5Dimer/mGluR5Total | 0.945 ± 0.064 | 0.603 ± 0.327 | ↓36% | ns |
| GABRβ3 / β-actin | 0.076 ± 0.029 | 0.048 ± 0.012 | ↓37% | 0.050 |
| GFAP/ β-actin | 0.087 ± 0.084 | 0.928 ± 0.491 | ↑967% | 0.029 |
| β-actin | 24.7 ± 3.17 | 22.9 ± 3.28 | ↓7.3% | ns |
| Age ± SD (years) | 32.6 ± 7.89 | 35.7 ± 10.9 | ↑9.5% | ns |
| PMI ± SD (years) | 21.5 ± 10.1 | 22.0 ± 8.91 | ↑2.3% | ns |
| Gender | 3M:2F | 7M:2F | -- | -- |
|
| ||||
| Children | Control | Autistic | Change | P |
|
| ||||
| FMRP/ β-actin | 0.422 ± 0.166 | 0.295 ± 0.263 | ↓30% | ns |
| mGluR5 Dimer/ β-actin | 0.099 ± 0.071 | 0.278 ± 0.077 | ↑181% | 0.0084 |
| mGluR5 Total/ β-actin | 0.086 ± 0.064 | 0.286 ± 0.071 | ↑232% | 0.0071 |
| mGluR5Dimer/mGluR5Total | 0.897 ± 0.058 | 0.972 ± 0.017 | ↑8.4% | 0.049 |
| GABRβ3 / β-actin | 0.057 ± 0.038 | 0.075 ± 0.036 | ↑32% | ns |
| GFAP/ β-actin | 0.156 ± 0.088 | 0.355 ± 0.101 | ↑128% | 0.031 |
| β-actin | 21.64 ± 3.41 | 20.13 ± 5.15 | ↓7% | ns |
| Age ± SD (years) | 10.7 ± 5.28 | 9.57 ± 4.28 | ↓11% | ns |
| PMI ± SD (years) | 19.6 ± 8.45 | 21.7 ± 11.3 | ↑11% | ns |
| Gender | 6M:0F | 5M:2F | -- | -- |
ns, not significant
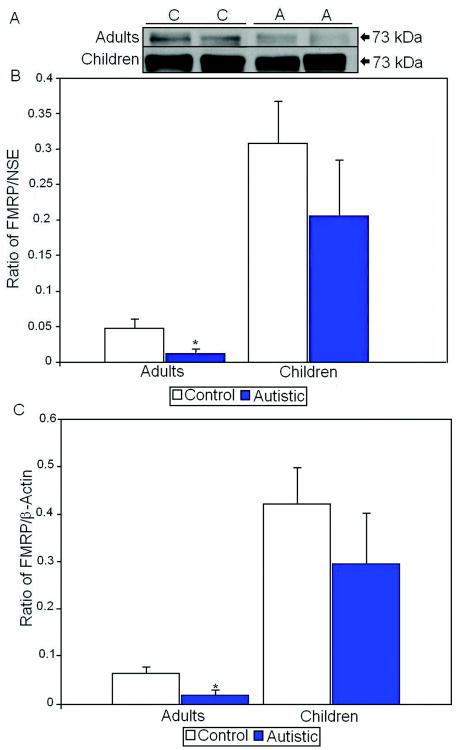
FMRP was visualized as an approximately 73 kDa doublet. There was a significant 75% reduction of FMRP/NSE (p<0.028; Table 2) and a 75% significant reduction of FMRP/ β-actin (p<0.038; Table 3) in adults with autism when compared with controls (Figure 2). In contrast, there were no significant differences in FMRP expression in children despite a trend for a reduction in FMRP levels (Figure 2).
Figure 2.
A. Representative samples of FMRP from control (C) and autistic (A) subjects. B. Mean FMRP/NSE ratios for autistic (filled histogram bars) and control subjects are shown for vermis. C. Mean FMRP/β-actin ratios for autistic (filled histogram bars) and control subjects are shown for vermis. (Error bars expressed as standard error of the mean.) *, p<0.05.
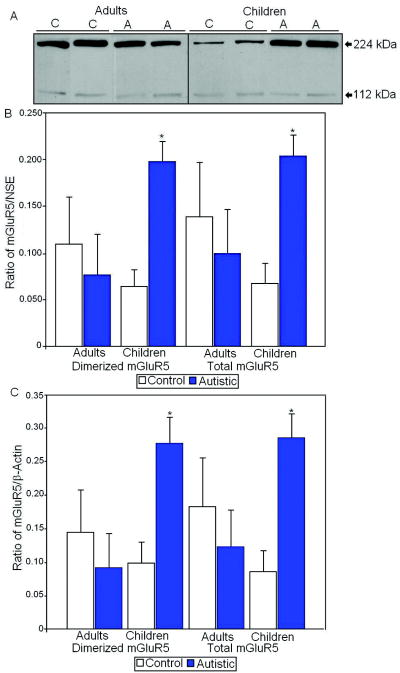
Metabotropic glutamate receptor 5 (mGluR5) appeared as a dimer at approximately 224 kDa and a monomer at 112 kDa (Figure 3). There was a significant 204% increase in the dimerized mGluR5/NSE (p<0.0023) and a 209% increase in total mGluR5/NSE (p<0.0042) in children with autism when compared with controls (Table 2, Figure 3). mGluR5/ β-actin dimer also displayed a significant 181% increase (p<0.0084) and total mGluR5/ β-actin was similarly significantly elevated by 232% (p<0.0071) in children with autism vs. controls (Table 3, Figure 3). There were no significant differences in mGluR5 expression in adults with autism vs. controls. We also measured the ratio of dimerized protein to total mGluR5 protein and found a significant increase in children with autism (p<0.049; Table 2).
Figure 3.
A. Representative samples of mGluR5 from control (C) and autistic (A) subjects. B. Mean mGluR5/NSE ratios for autistic (filled histogram bars) and control subjects are shown for vermis. C. Mean mGluR5/ β-actin ratios for autistic (filled histogram bars) and control subjects are shown for vermis. (Error bars expressed as standard error of the mean.) *, p<0.05.
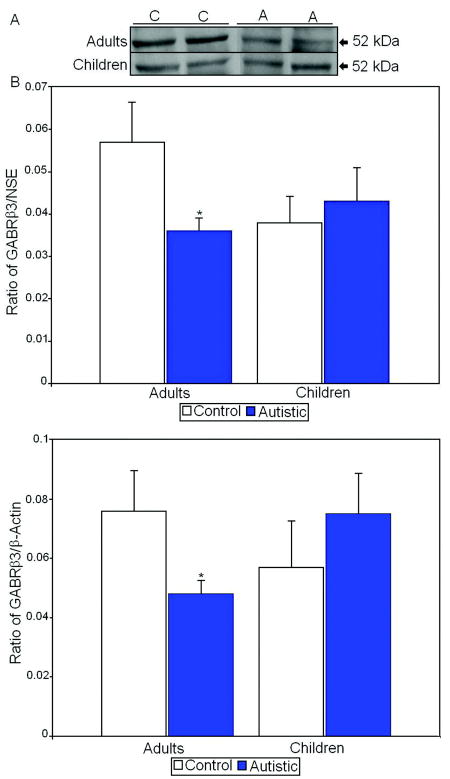
Since FMRP expression has been shown to affect expression of GABAA receptors in animal models (El Idrissi et al., 2005; D’Hulst et al., 2006; Gantois et al., 2006), we measured GABAA receptor beta 3 (GABRβ3) levels in children and adults with autism vs. controls. We found a significant 37% reduction in GABRβ3 levels in adults with autism when compared with healthy controls (p<0.031 for GABRβ3/NSE; p<0.05 for GABRβ3/ β-actin; Tables 2 and 3; Figure 4). In contrast we did not find any differences in GABRβ3 levels between autistic and control children (Tables 2 and 3; Figure 4).
Figure 4.
A. Representative samples of GABRβ3 from control (C) and autistic (A) subjects. B. Mean GABRβ3/NSE ratios for autistic (filled histogram bars) and control subjects are shown for vermis. C. Mean GABRβ3/ β-actin ratios for autistic (filled histogram bars) and control subjects are shown for vermis. (Error bars expressed as standard error of the mean.) *, p<0.05.
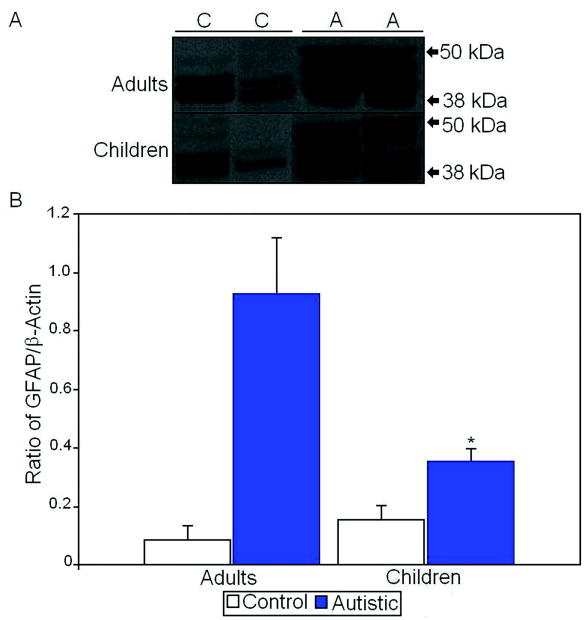
Finally, we also measured levels of glial fibrillary acidic protein (GFAP) as GFAP has previously been shown to be increased in multiple brain regions of subjects with autism (Laurence and Fatemi, 2005; Vargas et al., 2005). We found a 967% increase in GFAP levels in vermis of adults with autism when compared with controls (p<0.029; Table 3; Figure 5). Likewise, we found a 128% increase in GFAP protein expression in vermis of children with autism when compared with controls (p<0.031; Table 3; Figure 5).
Figure 5.
A. Representative samples of GFAP from control (C) and autistic (A) subjects. B. Mean GFAP/β-actin ratios for autistic (filled histogram bars) and control subjects are shown for vermis. (Error bars expressed as standard error of the mean.) *, p<0.05.
We examined the effects of confounds of age, sex, gender and PMI on our results and found no impact of these factors on levels of any proteins (data not shown).
Discussion
In the current study we have demonstrated for the first time significant reduction in levels of FMRP in the vermis of adults with autism as well as a nonsignificant reduction of FMRP in children with autism when compared with age and PMI matched controls. Additionally, we have shown for the first time that levels of mGluR5 protein is significantly increased in vermis of children with autism vs. age and PMI matched controls. Our results also show that levels of the same receptor are nonsignificantly reduced in adults with autism vs. controls. We have also demonstrated that the expression of mGluR5 in autistic children is significantly more homo-dimerized when compared with normal children. There was also a significant decrease in GABRβ3 in vermis of adult subjects with autism. Finally we found significant increases in GFAP in vermis of both children and adults with autism when compared with controls.
Significant reduction in FMRP in vermis of adults with autism is both novel and intriguing since none of the subjects used in this study had a diagnosis of FXS. This decrease in FMRP in vermis was also evident in children with autism, however, it did not reach statistical significance indicating that low power of our sample size may have contributed to this effect. The underexpression of FMRP in autistic subjects may be a universal event and could easily explain the presence of several potential endophenotypes that are shared between autism and FXS: 1) several autistic abnormalities including mental retardation, seizures, abnormal dendritic spine morphology, social anxiety, and reduced size of the cerebellar vermis (Hatton et al., 2006; Gothelf et al., 2008) are shared between both disorders; 2) valid animal models of FXS in mice and Drosophila melanogaster also show reduction in FMRP levels, GABAA receptor underexpression, behavioral, and glutamatergic receptor abnormalities (El Idrissi et al., 2005; D’Hulst et al., 2006; Gantois et al., 2006; Dölen et al., 2007); 3) Pak1 and Pak 3 (p21 associated tyrosine kinases) have been known to antagonize FMRP function (Hayashi et al., 2007) leading to changes in synaptic plasticity and abnormal spine morphology in animal models of FXS. These molecules may indeed be abnormal in autistic brain and their levels and functions are yet to be determined; 4) decreased FMRP levels have been associated with presence of increased colocalizable molecules such as calcium/calmodulin protein kinase II (CAMK2), activity regulated cytoskeleton-associated protein (ARC), and microtubule associated protein 1B (MAP1B) as well as homer (Antar et al., 2004; Lu et al., 2004; Irwin et al., 2005); 5) decreased FMRP is known to increase long term depression (LTD) (Bear et al., 2004) and increased epileptic discharges (Musumeci et al., 1999) as seen in autism; 6) decreased FMRP may also be associated with hypoplasia of cerebellar vermis especially since the same phenomenon has also been observed in subjects with FXS (Gothelf et al., 2008) who show a decrease in size of posterior cerebellar vermis; 7) multiple recent reports have also shown a decrease in size of cerebellar vermis in autism (Steinlin, 2008; Scott et al., 2009; Webb et al., 2009). All of these morphologic changes co-occurred with problems with language ability and cognitive abnormalities. Indeed, DeLorey et al., (2008) described GABRβ3 deficient mice who displayed hypoplasia of vermal lobules, and exhibited impaired exploratory and interactive behaviors, similar to what is observed in autism.
A second important and novel finding of the current study is the observation of significantly increased expression of mGluR5 in autistic children which has been unknown hitherto. It is quite interesting that the same receptor was non-significantly reduced in vermis of autistic adults when compared with controls. mGluR5 is a member of a group I metabotropic glutamate receptor system which modulates excitatory synaptic transmission and is involved in a number of important functions both during brain development and in adult life (Catania et al., 2007): 1) mGluR5 receptors are numerous at birth and show reductions in density later in life (Raol et al., 2001); 2) in rodents, mGluR5 receptors drop in number beyond postnatal day 18 and later in rat cerebellum (Romano et al., 1996); 3) mGlur5 receptors are present on stem cells that can give rise to neurons and glia and participate in basic developmental events that occur prior to synaptic formation such as during neuronal proliferation, differentiation and survival (Catania et al., 2007); 4) mGluR5 is also present on Cajal-Retzius cells, thus affecting the release of Reelin (Mienville, 1999; López-Bendito et al., 2002); 5) increase in mGluR5 in childhood may be responsible for early onset of seizures as seen in autism (Catania et al., 2007); 6) mGluR5 can protect against apoptosis action leading to increased cell number when activated (Copani et al., 1998); 7) Increased mGluR5 can lead to abnormal spine formation and abnormal synthesis of synaptic proteins most likely due to antagonism of FMRP (Grossman et al., 2006; Catania et al., 2007).
Abnormalities in expression of mGluRs have been observed in multiple neurological disorders including increased protein expression in Down’s syndrome (Oka and Takashima, 1999) and increased mRNA without change in protein levels in adult schizophrenia (Breese et al., 1995). mGluR5 has been reported by some investigators to appear on western blots as both a dimer of approximately 224-250 kDa as well as a monomer of approximately 112-130 kDa (Copani et al., 2000; Hermans and Challiss, 2001; Goudet et al., 2005). The dimerized form may represent a desensitized version of the receptor (Naur et al., 2005); however it has been assumed that the dimer form is the natural form of the receptor (Romano et al., 2001; Goudet et al., 2005; Schwendt and McGinty, 2007) and may be induced into dimerization by oxidative stress or via auto-induction (Copani et al., 2000). This is quite interesting since our data indicate that a significant proportion of mGluR5 expression seen in children with autism is in the dimer form, further supporting the hypothesis that activation of the receptor early in life (as it may occur in autism either because of oxidative stress or ischemia), could further initiate a vicious cycle of further dimerization and activation of additional mGluR5 receptors leading to a number of consequences that are related to furtherance of pathology observed in autism: 1) abnormal regulation of apoptosis in brain as reported by our laboratory and confirmed by others (Fatemi and Halt, 2001; Araghi-Niknam and Fatemi, 2003; Sheikh et al., 2010); 2) increase in frequency of seizures; 3) increased occurrence of LTD as well as abnormal conditioned eye blink response observed in animal models of autism (Bear et al., 2004); 4) decrease in number of GABA receptors; 5) potential increased expression of amyloid precursor protein as seen in Down’s Syndrome (Oka and Takashima,1999); 6) ultimate drop in mGluR5 expression in adults with autism as seen in this report may represent the final ending of a pathologic pathway that is observed in autism and is associated with decrease in long term potentiation (LTP), learning deficits, and LTD (Lu et al., 1997).
Our mGluR5 results in children with autism have to be analyzed with respect to sources for these receptors in cerebellar vermis. Group I mGluRs (consisting of mGluR1 and mGluR5), upon activation, couple to phospholipase C and regulate the IP3/Ca2+ signaling system (Knöpfel and Grandes, 2002). Quite interestingly, mGluR5 is localized to about 10% of the Golgi cells mainly in lobules I, II, VII-X of the cerebellar vermis (Neki et al., 1996; Négyessy et al., 1997) and to Lugaro cell, a type of inhibitory interneuron (Neki et al., 1996; Négyessy et al., 1997; Melik-Musyan and Fanardzhyan, 2004).
In rat cerebellar cortex, Négyessy et al., (1997) showed the presence of two splice variants of mGluR5 gene, namely mGluR5a and mGluR5b. These authors showed the presence of a range of molecular weight species, i.e., 128 kDa (mGluR5a), 132 kDa (mGluR5b) and a band of greater than 250 kDa as a dimeric form in the rat cerebella (Négyessy et al., 1997). These authors posit that activation of mGluR5 on Golgi cells might synchronize groups of granule cells to secrete Reelin. Supposedly, this activity of Golgi cells may lead to inhibition of Golgi cells releasing GABA as well as granule cells releasing Reelin. Both phenomena occur in autistic subjects (i.e. underproduction of Reelin and GABA in autism). Thus, both GABA and Reelin will be produced less in autistic cerebellum.
Neki et al., (1996) also showed that ≈ 8.8% of all Golgi cells carried mGluR5 receptors with 71% of these cells localized to vermis and 28% localized to hemispheric region of cerebellum. Thus, it appears clear that mGluR5 results obtained in this study reflect activation of mGluR5 localized to vermal Golgi and Lugaro cells.
There are, however, additional reports mostly supportive of the presence of mGluR5 on GFAP-bearing glial cells resident in vermis. Aronica et al., (2000) showed that status epilepticus (SE) resulted in hypertrophy of astrocytes and microglia along with an increase in mGluR5 protein expression in glial cells, which persisted up to three months after SE. Finally, Ferraguti et al., (2001) showed that administration of a subconvulsive dose of kainic acid to CA3 pyramidal cells led to proliferation of mGluR5 in glial cells which was positive by GFAP labeling. Thus, it appears that while the largest mGluR5 expression in subjects with autism is due to activation of these receptors resident on Golgi and Lugaro cells, presence of significant GFAP upregulation in the same tissue may also contribute to activation of these receptors. This scenario seems less likely since a further increase in GFAP in adults with autism did not match the activation or lack thereof in mGluR5 receptor levels in adults with autism.
The one dissenting report is by Tilleux et al., (2007) who showed downregulation of mGluR5 expression in cultured astrocytes treated with LPS, probably secondary to effects of tumor necrosis factor, since application of PGE2 and NO resulted in upregulation of mGluR5. The upregulation of mGluR5 in surviving neuronal cells is probably a consequence rather than a cause of the epileptic seizures as seen in TLE-resistant patients (Notenboom et al., 2006).
Our results with GABRβ3 reached statistical significance in adults with autism, while in children with autism we showed a trend for abnormality [i.e. elevation in children, possibly in response to a negative feedback loop; and significant reduction in adults as seen to be true in our previous published data with a different collection of brain samples with autism (Fatemi et al., 2009a)]. These results provide us with four potential avenues of treatment yet to be tested: 1) hyperactivation of mGluR5 in children with autism, at a time when receptors should not be as active, can be treated with negative allosteric modulators of the mGluR5 receptor tested in both animal models and recently in a group of patients with FXS (Berry-Kravis et al., 2009). This indicates that early treatment in children with autism may arrest the vicious cycle of mGluR5 activation, decreased FMRP and subsequent reduction in some or all GABAA receptors which can lead to permanent brain abnormalities including spine abnormalities and reduction in size of vermis; 2) in adult subjects with autism, when mGluR5 is reduced or at normal levels, it may be treated with positive allosteric modulators of the mGluR5 receptor which can improve cognition without provoking any seizures (Lecourtier et al., 2007) and are candidate treatments in schizophrenia; 3) in subjects with autism, use of drugs that mitigate the loss of FMRP on mGluR5-mediated translation (Bear et al., 2004; Berry-Kravis et al., 2008). An open label treatment trial of lithium in patients with FXS found improvement in total Aberrant Behavior Checklist score, Vineland Adaptive Behavior Scale, and Clinical Global Improvement Scale (Berry-Kravis et al., 2008). These results are consistent with results from animal models of FXS. Lithium reduces mGluR-mediated translation and it is hypothesized that repletion of FMRP has the same effect (Bear et al., 2004; Berry-Kravis et al., 2008); and finally; 4) Norbin, a neuronal protein, acts as a positive mediator of mGluR5 signaling and genetic deletion of norbin leads to altered LTP, LTD, and synaptic transmission (Wang et al., 2009). Treatment with norbin may reverse increased mGluR5 signaling in subjects with autism.
Acknowledgments
Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders, University of Maryland, Baltimore, MD (The role of the NICHD Brain and Tissue Bank is to distribute tissue, and therefore, cannot endorse the studies performed or the interpretation of results); the Harvard Brain Tissue Resource Center, which is supported in part by PHS grant number R24 MH068855; the Brain Endowment Bank, which is funded in part by the National Parkinson Foundation, Inc., Miami, Florida; and the Autism Tissue Program and is gratefully acknowledged. Grant support by National Institute of Child Health and Human Development (#5R01HD052074-01A2 and 3R01HD052074-03S1) to SHF is gratefully acknowledged. Assistance with statistical analysis from Dr. P. Thuras and Dr. G. Vazquez is acknowledged. Antibody information regarding FMRP from Dr. J. Darnell, Rockefeller University, is greatly appreciated.
Literature Cited
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: APA Press; 1994. [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carrol RC, Bassel GJ. Metabotropic glutamate receptor activation regulates Fragile X mental retardation protein and Fmr1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araghi-Niknam M, Fatemi SH. Levels of Bcl-2 and P53 are altered in superior frontal and cerebellar cortices of autistic subjects. Cell Mol Neurobiol. 2003;6:945–52. doi: 10.1023/B:CEMN.0000005322.27203.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Groter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Mesibov GB, Ament N, Skinner M. Early development, temperament, and functional impairment in autism and fragile X syndrome. J Autism Dev Disord. 2000;30:557–567. doi: 10.1023/a:1005412111706. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of Fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, Weiler IJ, Greenough WT. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J Dev Behav Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hessl D, Coffey S, Hervey C, Schneider A, Yuhas J, Hutchison J, Snape M, Tranfaglia M, Nguyen DV, Hagerman R. A pilot open label, single dose of fenobam in adults with Fragile X syndrome. J Med Genet. 2009;46:266–271. doi: 10.1136/jmg.2008.063701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ. GABAergic cerebellar system in autism: a neuropathological and developmental perspective. Int Rev Neurobiol. 2005;71:167–178. doi: 10.1016/s0074-7742(05)71007-2. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Fitzgerald CM, Guptill JT, Booker AB, Kemper TL, Bauman ML. Density and distribution of hippocampal neurotransmitter receptors in autism: an autoradiographic study. J Aut Dev Disord. 2001;31:537–544. doi: 10.1023/a:1013238809666. [DOI] [PubMed] [Google Scholar]
- Breese CR, Freedman R, Leonard SS. Glutamate receptor subtype expression in human postmortem brain tissue from schizophrenics and alcohol abusers. Brain Res. 1995;674:82–90. doi: 10.1016/0006-8993(94)01384-t. [DOI] [PubMed] [Google Scholar]
- Bureau M, Olsen RW. Multiple distinct subunits of the γ–aminobutyric acid-A receptor protein show different ligand-binding affinities. Mol Pharmacol. 1990;37:497–502. [PubMed] [Google Scholar]
- Catania MV, D’Antoni S, Bonaccorso CM, Aronica E, Bear MF, Nicoletti F. Group I metabotropic glutamate receptors: a role in neurodevelopmental disorders? Mol Neurobiol. 2007;35:298–307. doi: 10.1007/s12035-007-0022-1. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V, Musella A, Prosperetti C, Calabresi P, Bernardi G, Bagni C. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. 2008;63:963–973. doi: 10.1016/j.biopsych.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Cohen I. Behavioral profiles of autistic and nonautistic fragile X males. Dev Brain Dyn. 1995;8:252–269. [Google Scholar]
- Copani A, Casabona G, Bruno V, Caruso A, Condorelli DF, Messina A, Di Giorgi-Gerevini V, Pin J-P, Kuhn R, Knöpfel T, Nicoletti F. The metabotropic glutamate receptor mGlu5 controls the onset of developmental apoptosis in cultured cerebellar neurons. Eur J Neurosci. 1998;10:2173–2184. doi: 10.1046/j.1460-9568.1998.00230.x. [DOI] [PubMed] [Google Scholar]
- Copani A, Romano C, Di Giorgi Gerevini V, Nicosia A, Casabona G, Storto M, Mutel V, Nicoletti F. Reducing conditions differentially affect the functional and structural properties of group-I and –II metabotropic glutamate receptors. Brain Res. 2000;867:156–172. doi: 10.1016/s0006-8993(00)02293-9. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Pettay DL, Gold LB, Hersey SJ, Hinkle EF, Stanfield ML, Holmgreen P, Yeargin-Allsopp M, Boyle C, Sherman SL. Prevalence and phenotype consequence of FRAXA and FRAXE allele in a large ethnically diverse, special education-needs population. Am J Hum Genet. 1999;64:495–507. doi: 10.1086/302260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Fraser CE, Mostovetsky O, Darnell RB. Discrimination of common and unique RNA-binding activities of Fragile X mental retardation protein paralogs. Hum Mol Genet. 2009;18:3164–3177. doi: 10.1093/hmg/ddp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia or cerebellar vermal lobules: A potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, Bagni C. Fragile X mental retardation protein control of neuronal mRNA metabolism: Insights into mRNA stability. Mol Cell Neurosci. 2010;43:43–50. doi: 10.1016/j.mcn.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Shankaranarayana Rao BS, Smith GB, Auerbach D, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of Fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR. Altered levels of Bcl-2 and P53 proteins in parietal cortex reflect decreased apoptotic regulation in autism. Synapse. 2001;42:281–284. doi: 10.1002/syn.10002. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt A, Stary J, Kanodia R, Schulz SC, Realmuto G. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in parietal and cerebellar cortices of autistic subjects. Biol Psychiatry. 2002a;52:805–810. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Stary J, Egan EA. Reduced blood levels of Reelin as a vulnerability factor in the pathophysiology of autistic disorder. Cel Mol Neurosci. 2002b;22:139–152. doi: 10.1023/a:1019857620251. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Snow AV, Stary JM, Araghi-Niknam M, Reutiman TJ, Lee S, Brooks AI, Pearce D. Reelin signaling is impaired in autism. Biol Psychiatry. 2005;57:777–787. doi: 10.1016/j.biopsych.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Thuras PD. GABA(A) Receptor downregulation in brains of subjects with autism. J Autism Dev Disord. 2009a;39:223–230. doi: 10.1007/s10803-008-0646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD, Reutiman TJ, Thuras PD. Expression of GABA(B) receptors is altered in brains of Subjects with autism. Cerebellum. 2009b;8:64–69. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and Protein Levels for GABA(A) alpha 4, alpha 5, beta 1, and GABA(B)R1 Receptors are Altered in Brains from Subjects with Autism. J Autism Dev Disord. 2010 doi: 10.1007/s10803-009-0924-z. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersh SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Corti C, Valerio E, Mion S, Xuereb J. Activated astrocytes in areas of kainite-induced neuronal injury upregulate the expression of metabotropic glutamate receptors 2/3 and 5. Exp Brain Res. 2001;137:1–11. doi: 10.1007/s002210000633. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Past and future perspectives on autism epidemiology. In: Moldin SO, Rubenstein JLR, editors. Understanding autism from basic neuroscience to treatment. Boca Raton, FL: CRC/Taylor and Francis; 2006. pp. 25–48. [Google Scholar]
- Gantois I, Vandescompele J, Speleman F, Reyniers E, D’Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABAA receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis. 2006;21:346–357. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM. Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis. 2008;30:303–311. doi: 10.1016/j.nbd.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, Erba HW, Ringel J, Hayashi KM, Patnaik S, Golianu B, Kraemer HC, Thompson PM, Piven J, Reiss AL. Neuroanatomy of Fragile X syndrome is associated with aberrant behavior and the Fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudet C, Kniazeff J, Hlavackova V, Malhaire F, Maurel D, Acher F, Blahos J, Prézeau L, Pin J-P. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem. 2005;280:24380–24385. doi: 10.1074/jbc.M502642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AW, Aldridge GM, Weiler IJ, Greenough WT. Local protein synthesis and spine morphogenesis: Fragile X syndrome and beyond. J Neurosci. 2006;26:7151–7155. doi: 10.1523/JNEUROSCI.1790-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, Roberts J, Mirrett P. Autistic behavior in children with Fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet A. 2006;140A:1804–1813. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hayashi ML, Rao BS, Seo JS, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S. Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci USA. 2007;104:11489–11494. doi: 10.1073/pnas.0705003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Challiss RAJ. Structural, signaling and regulatory properties of the group I metabotropic glutamate receptors: Prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Christmon CA, Grossman AW, Galvez R, Kim SH, DeGrush BJ, Weiler IJ, Greenough WT. Fragile X mental retardation protein levels increase following complex environment exposure in rat brain regions undergoing active synaptogenesis. Neurobiol Learn Mem. 2005;83:180–187. doi: 10.1016/j.nlm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. J Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kauffman WE, Cortell R, Kau A, bukelis I, Tierney E, Gray R, Cox C, Capone G, Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. Am J Med Genet Part A. 2004;129A:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Keene JD. RNA regulons: Coordination of post-translational events. Nat Rev Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Grandes P. Metabotropic glutamate receptors in the cerebellum with a focus on their function in Purkinje cells. Cerebellum. 2002;1:19–26. doi: 10.1007/BF02941886. [DOI] [PubMed] [Google Scholar]
- Laurence JA, Fatemi SH. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 2005;4:206–210. doi: 10.1080/14734220500208846. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. 2007 doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Shigemoto R, Farién A, Luján R. Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex. 2002;12:625–638. doi: 10.1093/cercor/12.6.625. [DOI] [PubMed] [Google Scholar]
- Lu R, Wang H, Liang Z, Ku L, O’Donnell WT, Li W, Warren ST, Feng Y. The Fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci USA. 2004;101:14374–14378. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melik-Musyan AB, Fanardzhyan W. Morphological characteristics of Lugaro cells in the cerebellar cortex. Neurosci Behav Physiol. 2004;34:633–638. doi: 10.1023/b:neab.0000028297.30474.f9. [DOI] [PubMed] [Google Scholar]
- Mienville JM. Cajal-Retzius cells physiology: just in time to bridge the 20th century. Cereb Cortex. 1999;9:776–782. doi: 10.1093/cercor/9.8.776. [DOI] [PubMed] [Google Scholar]
- Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, De Sarro GB, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40:1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Naur P, Vestergaard B, Dkov LK, Egebjerg J, Gajhede M, Kastrup JS. Crystal structure of the kainite receptor GluR5 ligand-binding core in complex with (S)-glutamate. FEBS Lett. 2005;579:1154–1160. doi: 10.1016/j.febslet.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Négyessy L, Vidnyánszky Z, Kuhn R, Knöpfel T, Görcs TJ, Hámori J. Light and electron microscopic demonstration of mGluR5 metabotropic glutamate receptor immunoreactive neuronal elements in the rat cerebellar cortex. J Comp Neurol. 1997;385:641–650. doi: 10.1002/(sici)1096-9861(19970908)385:4<641::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Neki A, Oishi H, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Metabotropic glutamate receptors mGluR2 and mGluR5 are expressed in two non-overlapping populations of Golgi cells in the rat cerebellum. Neuroscience. 1996;75:815–826. doi: 10.1016/0306-4522(96)00316-8. [DOI] [PubMed] [Google Scholar]
- Notenboom RG, Hampson DR, Jansen GH, van Rijen PC, van Veelen CW, van Nieuwenhuizen O, de Graan PN. Up-regulation of hippocampal metabotropic glutamate receptor 5 in temporal lobe epilepsy patients. Brain. 2006;129:96–107. doi: 10.1093/brain/awh673. [DOI] [PubMed] [Google Scholar]
- Oblak A, Gibbs TT, Blatt GJ. Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Res. 2009;2:205–219. doi: 10.1002/aur.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka A, Takashima S. The up-regulation of metabotropic glutamate receptor 5 (mGluR5) in Down’s syndrome brains. Acta Neuropathol. 1999;97:275–278. doi: 10.1007/s004010050985. [DOI] [PubMed] [Google Scholar]
- Oostra BA, Willemsen R. FMR1: A gene with three faces. Biochem Biophys Acta. 2009;1790:467–477. doi: 10.1016/j.bbagen.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH, Wenk GL. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry. 2001;7:1058–1066. doi: 10.1176/appi.ajp.158.7.1058. [DOI] [PubMed] [Google Scholar]
- Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J. Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 2001;57:1618–1628. doi: 10.1212/wnl.57.9.1618. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lynch DR, Brooks-Kayal AR. Role of excitatory amino acids in developmental epilepsies. Ment Retard Dev Disabil Res Rev. 2001;7:254–260. doi: 10.1002/mrdd.1035. [DOI] [PubMed] [Google Scholar]
- Realmuto GM, Azeem MW. Autistic Disorder. In: Fatemi SH, Clayton P, editors. The Medical Basis of Psychiatry. Third Edition. New York: Humana Press; 2008. pp. 355–374. [Google Scholar]
- Romano C, Van den Pol AN, O’Malley KL. Enhance early developmental expression of the metabotropic glutamate receptor mGluR5 in rat brain: protein, mRNA, splice variants, and regional distribution. J Comp Neurol. 1996;367:403–412. doi: 10.1002/(SICI)1096-9861(19960408)367:3<403::AID-CNE6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Romano C, Miller JK, Hyrc K, Dikranian S, Mennerick S, Takeuchi Y, Goldberg MP, O’Malley KL. Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGluR5 dimerization. 2001 [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mol Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarto I, Wabnegger L, Dogl E, Sieghart W. Homologous sites of GABAA receptor α1, β3, γ2 subunits are important for assembly. Neuropharmacology. 2002;43:482–491. doi: 10.1016/s0028-3908(02)00160-0. [DOI] [PubMed] [Google Scholar]
- Schwendt M, McGinty JF. Regulator of G-protein signaling 4 interacts with metabotropic glutamate receptor 5 in rat striatum: Relevance to amphetamine behavioral sensitization. J Pharmacol Exp Ther. 2007;323:650–657. doi: 10.1124/jpet.107.128561. [DOI] [PubMed] [Google Scholar]
- Scott JA, Schumann CM, Goodlin-Jones BL, Amaral DG. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Res. 2:246–257. doi: 10.1002/aur.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh AM, Li X, Wen G, Taqeer D, Brown WT, Malik M. Cathespin D and apoptosis related proteins are elevated in the brain of autistic subjects. Neuroscience. 2010;165:363–370. doi: 10.1016/j.neuroscience.2009.10.035. [DOI] [PubMed] [Google Scholar]
- Steinlin M. Cerebellar disorders in childhood: Cognitive problems. Cerebellum. 2008;7:607–610. doi: 10.1007/s12311-008-0083-3. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Berger J, Hermans E. Induction of astrogliosis by activated microglia is associated with a down-regulation of metabotropic glutamate receptor 5. J Neuroimmunol. 2007;189:23–30. doi: 10.1016/j.jneuroim.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- Wang H, Westin L, Nong Y, Birnbaum S, Bendor J, Brismar H, Nestler E, Aperia A, Flajolet M, Greengard P. Norbin in an endogenous regulator of metabotropic glutamate receptor 5 signaling. Science. 2009;326:1554–1557. doi: 10.1126/science.1178496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Sparks BF, Friedman SD, Shaw DW, Giedd J, Dawson G, Dager SR. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 2009;172:61–67. doi: 10.1016/j.pscychresns.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip J, Soghomonian JJ, Blatt GJ. Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathologica. 2007;113:559–568. doi: 10.1007/s00401-006-0176-3. [DOI] [PubMed] [Google Scholar]