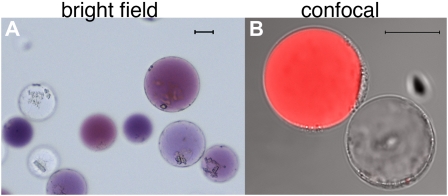
Figure 1.
Protoplasts freshly isolated from petals of the petunia hybrid M1XV30. A, Bright-field image. The two cell types are recognizable by the presence of anthocyanins in the central vacuole of epidermal cells and their absence in the mesophyll cells. B, Confocal image of the same protoplast preparation as in A. The red fluorescence, due to anthocyanin autofluorescence, allows the recognition of the two cell types (both visible in transmitted light) during confocal analysis. The size bar equals 20 μm. Protoplast isolation: Petals or leaves from greenhouse-grown plants were sterilized in 5% hypochlorite solution (for 30 s then rinsed in sterile water) and perforated using a needle bed (a “kenzan” for Japanese ikebana), prior overnight digestion in TEX buffer (B5 salts, 500 mg/L MES, 750 mg/L CaCl2 [2*H2O] 250 mg/L NH4NO3, and 0.4 m Suc [13.7%], pH 5.7), plus 0.2% Macerozyme R10 and 0.4% Cellulase R10 (Yakult). The digested material was filtered through a 150-SIGMA mesh filter (or similar filter) and protoplast suspension was then centrifuged for 10 min at 75g at room temperature in a swing-out rotor to concentrate the protoplasts in a band floating above the medium. After 2× washing with 10 mL of TEX buffer (centrifugation at 75g for 5 min between washing steps) protoplasts were then resuspended in an appropriate volume of MMM solution (0.5 m mannitol, 15 mm MgCl2, 0.1% MES). A total of 300 μL of protoplasts was used for each transformation: 30 μg of (supercoiled) plasmid DNA was added followed by 300 μL of polyethylene glycol solution [0.4 m mannitol, 0.1 m Ca(NO3)2*H2O, 40% polyethylene glycol brought to pH 8.0 with KOH] and 2 mL of TEX. Incubation at 25°C for 2 h was followed by washing with TEX buffer, as described before, and resuspended, after centrifugation, in 2 mL of TEX buffer. We have applied this protocol to flowers of different ages (from nearly open buds to fully expanded petals) and genotypes (different genetic backgrounds and/or mutations in genes affecting pigment deposition and/or vacuolar acidification). Transformation efficiency was in all cases above 60%. Plants were grown in a greenhouse with temperature never below 19°C and never exceeding 30°C, with a cycle of a minimum of 16 h of light in all seasons (supplied with artificial light in the winter). Suboptimal or unstable plant growth conditions can make efficiency of protoplast isolation and transformation drop dramatically.