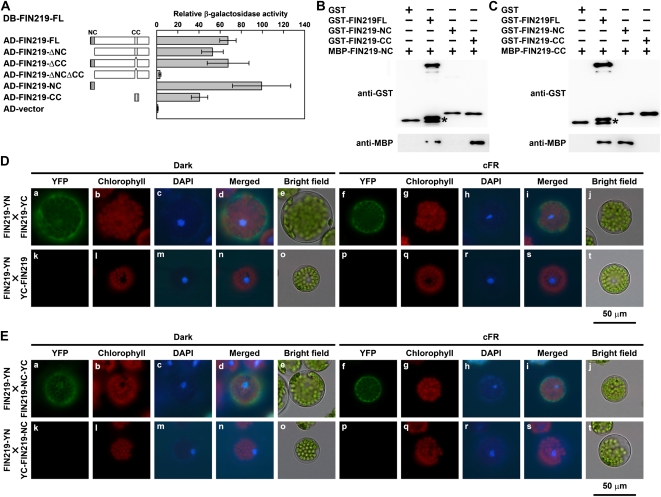
Figure 4.
FIN219 is able to interact with itself in vitro and in vivo. A, Interaction assay of FIN219 and FIN219 by the yeast LexA two-hybrid system. The value of interaction by β-galactosidase activity was the average of five individual yeast colonies. Error bars represent sd. The level of activation domain (AD) vector was arbitrarily set to 1. FIN219-ΔNC, FIN219FL minus the N-terminal coiled-coil domain; FIN219-ΔCC, FIN219FL minus the C-terminal coiled-coil domain; FIN219-ΔNCΔCC, FIN219FL minus the N-terminal coiled-coil domain and the C-terminal coiled-coil domain; FIN219-NC, the N-terminal coiled-coil domain of FIN219; FIN219-CC, the C-terminal coiled-coil domain of FIN219. DB, DNA-binding domain. B, The N-terminal coiled-coil domain of FIN219 can bind with the C-terminal coiled-coil domain on pull-down assay. Different recombinant proteins, including FIN219 fused with a GST tag (GST-FIN219FL, GST-FIN219-NC, and GST-FIN219-CC) or with maltose-binding peptide (MBP-FIN219-NC) purified from E. coli, were used. MBP-FIN219-NC was mixed with various recombinant proteins indicated above the blots and immunoprecipitated with GST resins. The immunoprecipitated products were probed with GST (top panel) and MBP (bottom panel) antibodies. The asterisk indicates a breakdown product of GST-FIN219FL. C, The interaction of the N-terminal coiled-coil domain of FIN219 and the C-terminal coiled-coil domain was confirmed by switch-tag fused proteins. The C-terminal coiled-coil domain of FIN219 fused with MBP (MBP-FIN219-CC) was mixed with various recombinant proteins indicated above the blot and used for pull-down assay as shown in B. The asterisk indicates a breakdown product of GST-FIN219FL. D and E, Assays of the interaction of the full-length FIN219 and itself or its N-terminal coiled-coil domain (FIN219-NC) by BiFC. The BiFC plasmids containing the full-length FIN219 and itself or FIN219-NC were cotransfected into Arabidopsis mesophyll protoplasts isolated from fin219-2. The transfected protoplasts were cultured overnight under darkness (a–e and k–o) or cFR light (f–j and p–t). The yellow fluorescent protein (YFP) channel is shown as the interaction signal of BiFC, the chlorophyll channel as autofluorescence of chlorophyll, the 4′,6-diamidino-2-phenylindole (DAPI) channel as the nucleus with DAPI staining, and the merged channel as the combination of YFP, chlorophyll, and DAPI channels.