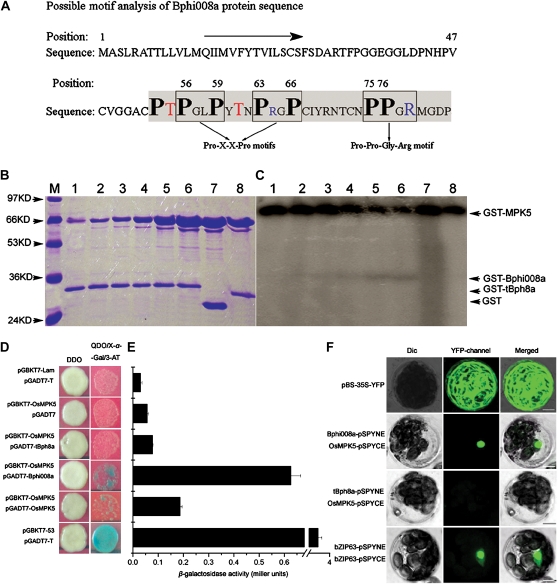
Figure 6.
Bphi008a interacts with OsMPK5 in vitro and in vivo. A, Possible motifs of the Bphi008a protein. Enclosed squares are two possible Pro-X-X-Pro motifs and one Pro-Pro-Gly-Arg motif. Bold black letters represent Pro residues in possible motifs; red letters represent possible Thr phosphorylated sites before the Pro-X-X-Pro motifs; blue letters represent positively charged Arg in motifs. The shaded region of the C terminus was also cleaved, and the remaining region was used as a negative control. B and C, Phosphorylation of the Bphi008a by OsMPK5 in vitro. Lanes 1 to 6, Kinase activities obtained from incubating 3 μg of GST-Bphi008a protein with 0.5, 1, 2, 4, 8, and 16 μg of GST-OsMPK5 protein, respectively, in an in vitro assay; lanes 7 and 8, kinase activities obtained from incubating 3 μg of GST and GST-tBph8a protein, respectively, with 16 μg of GST-OsMPK5 protein in an in vitro assay; lane M, protein masses in kD. D, Yeast two-hybrid assay of the interaction between Bphi008a or tBph8a and OsMPK5. The diploids were grown on DDO plates and transferred to QDO/X-α-Gal/3-amino-1,2,4-triazole (3-AT) plates with 20 μg mL−1 X-α-Gal and 10 μm 3-AT for 3 d. E, Relative β-galactosidase activities obtained were consistent with the results observed on QDO/X-α-Gal/3-AT plates. F, BiFC visualization of the Bphi008a-OsMPK5 and tBph8a-OsMPK5 interaction in transiently coexpressed Arabidopsis mesophyll protoplasts. The pBS-35S-YFP vector served as a transfected control, and the Arabidopsis nuclear protein bZIP63 served as a BiFC positive control. Dic, Differential interference contrast. Bars = 10 μm.