Abstract
The UNC-6/netrin guidance cue functions in axon guidance in vertebrates and invertebrates, mediating attraction via UNC-40/DCC family receptors and repulsion via by UNC-5 family receptors. The growth cone reads guidance cues and extends lamellipodia and filopodia, actin-based structures that sense the extracellular environment and power the forward motion of the growth cone. We show that UNC-6/netrin, UNC-5 and UNC-40/DCC modulated the extent of growth cone protrusion that correlated with attraction versus repulsion. Loss-of-function unc-5 mutants displayed increased protrusion in repelled growth cones, whereas loss-of-function unc-6 or unc-40 mutants caused decreased protrusion. In contrast to previous studies, our work suggests that the severe guidance defects in unc-5 mutants may be due to latent UNC-40 attractive signaling that steers the growth cone back towards the ventral source of UNC-6. UNC-6/Netrin signaling also controlled polarity of growth cone protrusion and F-actin accumulation that correlated with attraction versus repulsion. However, filopodial dynamics were affected independently of polarity of protrusion, indicating that the extent versus polarity of protrusion are at least in part separate mechanisms. In summary, we show here that growth cone guidance in response to UNC-6/netrin involves a combination of polarized growth cone protrusion as well as a balance between stimulation and inhibition of growth cone (e.g. filopodial) protrusion.
Keywords: Filopodia, Growth cone, Lamellipodia, Protrusion, UNC-6/netrin
INTRODUCTION
Developing axons are led by growth cones, dynamic actin-based structures that sense and respond to extracellular cues, driving the forward motion of the axon (Mortimer et al., 2008; Tessier-Lavigne and Goodman, 1996). Growth cones consist of lammelipodial protrusions of a branched actin meshwork and filopodial protrusions of bundled actin filaments, which are both involved in the proper growth of an axon to its target destination (Gallo and Letourneau, 2004; Pak et al., 2008; Zhou and Cohan, 2004).
The laminin-like UNC-6/netrin guidance cue and its receptors UNC-40/DCC (deleted in colorectal carcinoma) and UNC-5 have been widely implicated in axon pathfinding in mouse, Drosophila, C. elegans and other organisms (Chan et al., 1996; Hong et al., 1999; Leonardo et al., 1997; Montell, 1999; Pan et al., 2010; Shekarabi and Kennedy, 2002; Moore et al., 2007). In C. elegans, UNC-6 is secreted by ventral cells and is necessary for guidance of circumferential neurons (Ishii et al., 1992). The receptor UNC-40 is expressed in growth cones and mediates an attractive response to UNC-6, driving the ventral guidance of circumferential axons, while the receptor UNC-5 is expressed in dorsally guided growth cones and mediates a repulsive response to UNC-6, driving the dorsal guidance of circumferential axons (Hedgecock et al., 1990; Hong et al., 1999).
Vertebrate DCC and UNC-5 both respond to netrin by the formation of receptor dimers. In neurons expressing only DCC, netrin binding causes the formation of an DCC homodimer, leading to an attractive response by the growth cone. In neurons expressing both UNC-5 and DCC, netrin binding leads primarily to UNC-5 alone or UNC-5 and DCC signaling, both of which mediate a repulsive response (Hong et al., 1999; MacNeil et al., 2009).
Whereas the role of UNC-6/netrin and its receptors have been studied extensively in axon pathfinding endpoint analyses and in vitro growth cone experiments, little is known about how they affect the growth cone in vivo during growth cone outgrowth. Previous studies have shown that UNC-6/netrin can affect the polarity of growth cone protrusions in C. elegans (Adler et al., 2006), and the morphology of the growth cone in neurons in vitro (Shekarabi and Kennedy, 2002; Shekarabi et al., 2005). This led us to speculate that UNC-6/netrin and its receptors may play an important role in modulating both the extent and the polarity of protrusion in C. elegans growth cones.
We endeavored to understand how UNC-6/netrin and its receptors UNC-40/DCC and UNC-5 affect axon pathfinding, growth cone morphology and dynamics in circumferential neurons in C. elegans, using methods we have previously developed that allow in vivo analysis of C. elegans growth cones. We show here that UNC-6/netrin and its receptors UNC-40/DCC and UNC-5 are involved in both polarity of growth cone protrusiveness as well as in modulating the extent of protrusion. Indeed, these molecules affected filopodial dynamics independently of their polarity of protrusion, suggesting that polarity of protrusion and extent of protrusion might be governed at least in part by separate mechanisms and that growth cone guidance in vivo involves a combination of the two. Our results also suggest that UNC-5, UNC-6 and UNC-40 regulate a balance of protrusion within a growth cone, with UNC-6 driving protrusion via UNC-40 and inhibiting protrusion via UNC-5. This balance might establish a dorsal-ventral gradient of protrusion across the growth cone, which in part might underlie growth cone polarity and directed outgrowth.
MATERIALS AND METHODS
Genetic methods
Experiments performed at 20°C using standard C. elegans techniques (Brenner, 1974). These mutations and transgenics were used: X, unc-6(ev400 and e78), lqIs170 [rgef-1::vab-10ABD::gfp]; I, unc-40(n324 and e1430), unc-73(rh40); II, juIs76 [unc-25::gfp]; IV, kyIs179 [unc-86::gfp], unc-5(e53 and e152);?, lqIs128 [unc-25::myr::unc-40], lqIs129 [unc-25::myr::unc-40], lqIs164 [myo-3::cfp], lqIs182 [unc-86::myr::gfp] and lhIs6 [unc-25::mCherry]. Extrachromosomal arrays were attained by injection into the germline, and then integrated into the genome via standard techniques (Mello and Fire, 1995). Double mutants with unc-6, unc-5 and unc-40 were confirmed by the uncoordinated movement phenotype, axon pathfinding phenotype and PCR genotyping.
Imaging of axon guidance defects
VD neurons were visualized with an unc-25::gfp (Jin et al., 1999) or unc-25::mCherry transgene, expressed in all GABAergic neurons, including the 16 VDs. VD axons were considered defective if the axon failed to reach the dorsal nerve cord or if it branched or turned at an angle greater than 45° before reaching the dorsal nerve cord. To determine severity of defects, a myo-3::cfp transgene was introduced to mark the ventral and dorsal muscle quadrants. Using an unc-25::mCherry construct we could then determine whether an the trajectory of an axon went awry before or after reaching the dorsal edge of the ventral muscle quadrant.
HSNs were visualized with an unc-86::gfp transgene (Shen and Bargmann, 2003), which is expressed in numerous neurons, including the two HSNs. HSN axons were considered defective if the axon failed to reach the ventral nerve cord, or if it branched or turned at an angle greater than 45° before reaching the ventral nerve cord.
Growth cone time-lapse imaging
VD growth cones were imaged as previously described (Norris et al., 2009). Briefly, animals harboring the juIs76 [unc-25::gfp] transgene were selected 16 hours post-hatching at 20°C and placed on a 2% agarose pad with a drop of 10 mM muscimol (Sigma-Aldrich, St Louis, MO, USA) in M9 (Weinkove et al., 2008), which was allowed to evaporate for 3-4 minutes before placing coverslip on top of the animals on the pad. Growth cones were imaged with a Qimaging Rolera mGi EMCCD camera using a very short exposure time (∼30 mseconds) on a Leica DMR microscope. Images were acquired at intervals of 120 seconds, with total duration of time lapse ranging from 20 to 60 minutes. HSN growth cone time-lapse imaging was performed identically, except that animals were selected at 19 hours post hatching.
Growth cone protrusions were scored as previously described (Norris et al., 2009). Dynamic projections less than 0.5 μm in width emanating from the growth cone were scored as filopodia; protrusions greater than 0.5 μm were considered lamellipodial extensions; and protrusions that were static were not considered filopodia. Filopodia were considered to have disappeared at the point when there was no longer a visible a protrusion of less than 0.5 μm protruding from the membrane at the location where the filopodium had once been. Filopodia length and growth cone area were measured using ImageJ software. Filopodia length was determined by drawing a straight line from the base where the filopodium originates on the edge of the peripheral membrane to the tip of the filopodium. Polarity of filopodia protrusion was determined using ImageJ and dividing the growth cone into roughly four equally sized quadrants (dorsal-anterior, dorsal-posterior, ventral-anterior and ventral-posterior) and determining from which quadrant each filopodium emanated (see Fig. 7B for example). Significance of difference was determined in all cases by a two-sided t-test with unequal variance or a Fisher's exact test.
Fig. 7.
Netrin signaling mutants cause growth cone polarity defects. (A) Percentage of filopodia protruding dorsally from VD growth cones. The x-axis is set at 50%, such that bars extending above the x-axis represent percentages above 50%, and bars that extend below the axis represent percentages below 50%. Asterisks indicate a significant difference between wild type and the mutant (P<0.01) determined by two-sided t-test with unequal variance. (B,C) Representative images of wild-type and unc-5(e53) growth cones, with a line drawing of the growth cone and filopodial perimeter on the right. Scale bar: 5 μm for B,C,F,G. Dashed gray lines in B delineate dorsal and ventral boundaries of the growth cone; solid lines indicate the dorsal-ventral and anterior-posterior boundaries used for scoring filopodia polarity. (D) Graphical representations of relative filopodial protrusion from each quadrant of the VD growth cones (dorsal left, dorsal right, ventral right, ventral left), generated by the Spoke12 program (M. Tourtellot, University of Kansas). Dorsal is upwards and anterior is towards the left. (E) Percentage of filopodia protruding dorsally from HSN growth cones, as described in A. (F,G) Representative images of wild-type and unc-40(n324) growth cones, with arrows marking growth cone protrusions. (H) Relative filopodial protrusion from each quadrant of HSN growth cones as described in D.
VAB-10ABD::GFP for F-actin visualization
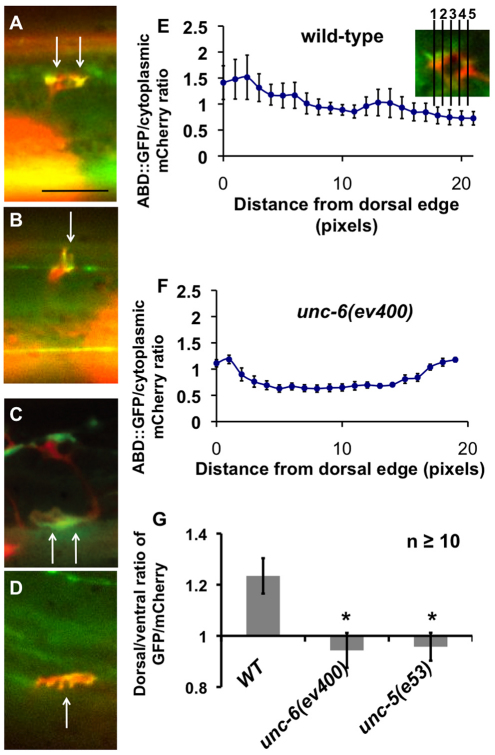
F-actin was visualized by imaging growth cones expressing the F-actin binding domain of spectraplakin VAB-10 fused to GFP, which has previously been used to monitor F-actin in other C. elegans cells (Bosher et al., 2003; Patel et al., 2008). We compared localization of this construct to the localization of a cytoplasmic mCherry to control for nonspecific factors such as variable growth cone size and shape. Asymmetric accumulation was quantified with line scans through the growth cone on the dorsal-ventral axis using NIH Image (see Fig. 8). Five line scans were made through each growth cone, and the average pixel intensity of the green channel was divided by the average intensity of the red channel to yield a relative intensity ratio indicating the level of enrichment of the VAB-10ABD::GFP construct.
Fig. 8.
Netrin signaling mutants cause loss of growth cone F-actin polarity. (A-D) Representative images of VD growth cones with cytoplasmic mCherry in red (a volumetric marker) and the VAB-10ABD::GFP in green. Areas of overlap are yellow. Scale bar: 5 μm. (A,B) In wild type, VAB-10ABD::GFP was predominantly at the dorsal edge of the growth cones (arrows). (C,D) Representative images of unc-6(ev400) VD growth cones with cytoplasmic mCherry and VAB-10ABD::GFP expression. Both growth cones have undergone guidance errors. In C, the growth cone first grew dorsally, then back ventrally, and is shown growing laterally. The growth cone in D is advancing laterally. Dorsal accumulation of VAB-10ABD::GFP is not observed in either growth cone. The growth cone in C displayed a ventral accumulation, and the growth in D displayed no asymmetric accumulation. (E,F) Representative line plots of a wild-type VD growth cone (E) and an unc-6(ev400) mutant growth cone (F) with VAB-10ABD::GFP expression. The x-axis represents distance (in pixels) from the dorsal edge of the growth cone of a line drawn vertically from the dorsal growth cone to the ventral growth cone (see inset). The y-axis represents the relative pixel intensity of VAB-10ABD::GFP compared with the volumetric mCherry marker (GFP/mCherry). Five lines were drawn for the growth cone and the pixel intensity ratios were averaged. At these exposures, the GFP and mCherry intensities were comparable, such that the ratios were close to 1. These graphs represent data from single growth cones, but others show a similar pattern. Error bars represent s.e.m. of the intensity ratios of the five line scans. (G) The average dorsal-to-ventral ratio of GFP/mCherry from multiple growth cones. This graph was generated using line scans on multiple growth cones as described in E and F. Growth cones were divided into dorsal and ventral halves of identical area, and the average intensity ratio of VAB-10ABD::GFP/mCherry was determined for each half. The dorsal ratios were divided by the ventral ratios to determine the relative dorsal enrichment of VAB-10ABD::GFP. A mean and s.e.m. from multiple growth cones was generated and plotted. n≥10; *P≤0.05.
RESULTS
UNC-5, UNC-6 and UNC-40 control dorsal-ventral axon pathfinding
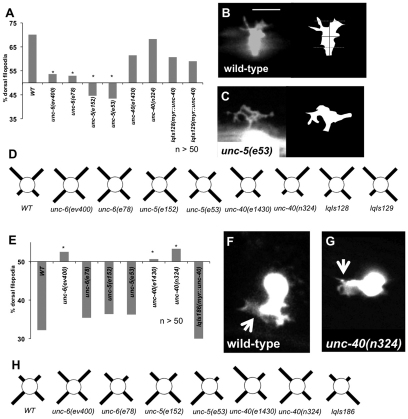
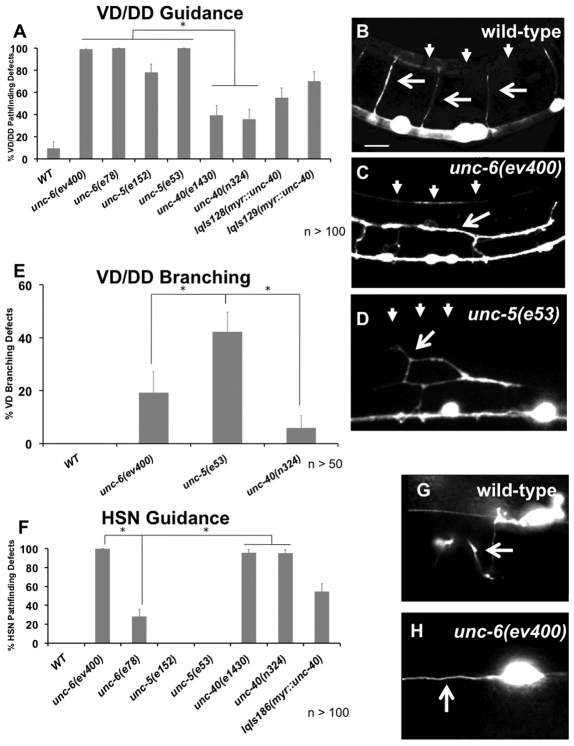
In C. elegans, unc-6 and unc-40 mutants display guidance defects in both ventrally directed and dorsally directed circumferential axons, and unc-5 mutants display defective pathfinding in dorsally directed circumferential axons (Hedgecock et al., 1990). We confirmed and quantified defects in unc-5, unc-6 and unc-40 mutants in the dorsally directed VD motoneurons that are repelled from UNC-6, and in the ventrally directed HSN axons that are attracted to UNC-6 (Fig. 1). Consistent with previous results, unc-5 and unc-6 mutants displayed a nearly complete failure of VD axons to reach the dorsal nerve cord, whereas unc-40 mutants had a weaker effect on VD axon pathfinding (Fig. 1A-D). By contrast, unc-6 and unc-40 mutants had strong HSN axon guidance defects, whereas unc-5 mutants did not (Fig. 1F-H). The unc-6(e78) allele, which results in a cysteine to tyrosine substitution in domain V-3 that disrupts interaction with UNC-5 but not UNC-40/DCC (Lim and Wadsworth, 2002), more strongly affected dorsal repulsive growth than ventral attractive growth, as previously reported (Fig. 1A,F). These results are consistent with previous findings that UNC-6/netrin and UNC-40/DCC, but not UNC-5, are required for ventral migration (Hedgecock et al., 1990).
Fig. 1.
Netrin signaling mutants cause axon pathfinding defects. (A) Percentage of VD/DD axons with pathfinding defects. (B-D) Fluorescent micrograph of L4 VD/DD axons; (B) normal pathfinding in wild-type axons (arrows) that reach the dorsal nerve cord (arrowheads). (C) Misguided and prematurely stopped axons (arrow) that do not reach the DNC (arrowheads) in unc-6(ev400). (D) An ectopic axon branch in unc-5(e53) (arrow). Arrowheads indicate axons. (E) Percentage of VD/DD axons with obvious ectopic axon branches. (F) Percentage of HSN axons with pathfinding defects. (G,H) Fluorescent micrographs of wild-type and unc-6(ev400) HSN axons; (G) normal pathfinding in wild type; and (H) failure to migrate ventrally in unc-6(ev400). Scale bar: 5 μm. Asterisks indicate significant difference between genotypes (P<0.01) determined by Fisher's exact test, and all genotypes were significantly different from wild type. Error bars represent 2× standard error of the proportion.
UNC-5 and UNC-6 inhibit growth cone protrusion
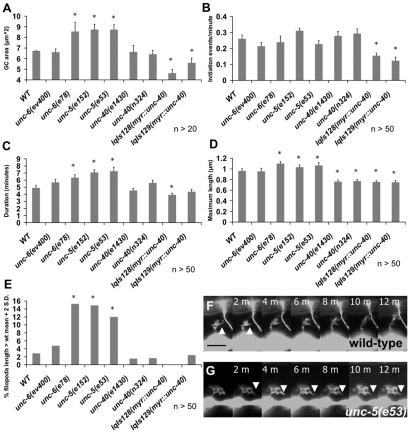
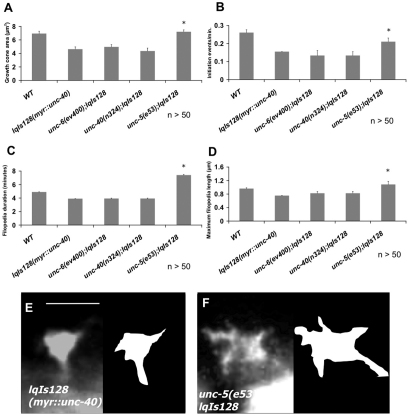
Although it is established that UNC-6/netrin signaling affects circumferential axon guidance, the effects of these molecules on the growth cone during outgrowth in vivo are less well understood. We imaged the growth cones of the VD motoneurons that are repelled from UNC-6/netrin over time as they extended. VD growth cones were imaged in animals at 16 hours post-hatching at a time when the VD growth cones have begun their commissural migrations (see Materials and methods for imaging methods). We measured growth cone area as well as the dynamic parameters of filopodia initiation rate, filopodia duration and maximal filopodial length (see Materials and methods for parameter measurement). Wild-type VD growth cones were dynamic (see Movie 1 in the supplementary material) and had an average area of 6.7 μm2 (Fig. 2A). Wild-type growth cones also displayed multiple dynamic filopodia that formed at an average rate of 0.26 per minute (one initiation event every 3.8 minutes) (Fig. 2B,F). The average wild-type filopodium had a duration of 4.9 minutes (between the time it was first noticeable to the time it was no longer apparent) (Fig. 2C) and had an average maximal length of 0.96 μm (Fig. 2D). These parameters are similar to those previously observed for wild-type VD growth cones (Norris et al., 2009).
Fig. 2.
Netrin signaling mutants affect VD growth cone morphology and filopodial dynamics. (A-D) Quantification of filopodia dynamics in VD growth cones. (A) Growth cone area in μm2. (B) Filopodia formation rate, in initiation events per minute. (C) Duration of filopodia once formed, in minutes. (D) Maximum filopodia length, in μm. (E) Percentage of filopodia greater than the wild-type average + 2 standard deviations in length. Error bars represent 2× standard error of the mean; asterisks indicate the significant difference between wild-type and the mutant genotype (P<0.01) determined by two-sided t-test with unequal variance. (F,G) Timelapse series of live growth cones, taken at 2 minutes per frame. (F) A wild-type VD growth cone. (G) An unc-5(e53) growth cone. Arrowheads indicate representative filopodia. Scale bar: 5 μm.
To understand how axonal repulsion relates to growth cone morphology during outgrowth, we analyzed VD growth cones in unc-5 mutants. unc-5 caused an increase in growth cone protrusiveness in VD growth cones (see Movie 2 in the supplementary material). Growth cone area was significantly increased (Fig. 2A,G), as was filopodia duration (Fig. 2C). Average maximal filopodial length was also increased significantly (Fig. 2D). However, filopodial length appeared biphasic in unc-5 mutants, with many filopodia that were comparable with wild-type length and some that grew very long (greater than two standard deviations from average wild-type length) (Fig. 2E,G; see Movie 1 in the supplementary material). Some of these long filopodia were observed to endure throughout the growth cone imaging period, suggesting that they are very stable growth cone protrusions not observed in wild type. These data indicate that UNC-5 is normally required to limit the extent of protrusion of growth cones that are repelled from UNC-6. unc-5 mutants did not affect the rate of filopodia formation (Fig. 2B), suggesting that UNC-5 might affect filopodial dynamics after a filopodium has been formed.
Surprisingly, the unc-6(ev400) null mutation had no significant effect on any of the VD growth cone parameters we measured (Fig. 2A-E). However, the unc-6(e78) mutation that specifically disrupts the repulsive, UNC-5-dependent role of UNC-6 (Lim and Wadsworth, 2002), resembled unc-5 mutants in all parameters measured (increased growth cone area, increased filopodial duration and unusually long filopodia with no effect on filopodia initiation rate) (Fig. 2A-E). That these effects are reduced in the unc-6 null suggests that in unc-6(e78), increased growth cone protrusiveness was due to a non-UNC-5 mediated role of UNC-6, possibly interaction with UNC-40. Indeed, as we show below, loss of UNC-40 suppressed the increased filopodial dynamics in unc-5 mutants and in unc-6(e78), indicating that UNC-40 is required for increased growth cone protrusion in these mutants.
Constitutively-active MYR::UNC-40 inhibits VD growth cone protrusion
Two putative null mutations in unc-40, e1430 and n324, had very little effect on VD growth cone dynamics and morphology (Fig. 2A-E), with the only significant effect being a reduction in maximal filopodial length compared with wild type (Fig. 2D). This is surprising given that UNC-40 is thought to cooperate with UNC-5 in repulsive axon guidance.
To probe the role of UNC-40 in VD growth cone dynamics in more detail, we constructed a constitutively active version of UNC-40, based upon Gitai et al. (Gitai et al., 2003); by adding an N-terminal myristoylation signal to the cytoplasmic domain of UNC-40. MYR::UNC-40 lacks the transmembrane domain and the extracellular domains, which function to inhibit dimerization in the absence of UNC-6/netrin. MYR::UNC-40 is targeted to membranes by the myristoylation sequence and is thought to be constitutively active even in the absence of UNC-6/netrin.
Expression of myr::unc-40 was driven in the VD neurons using the unc-25 promoter. In a wild-type background, MYR::UNC-40 caused a marked decrease in VD growth cone protrusiveness (see Movie 3 in the supplementary material). MYR::UNC-40 growth cones were small [6.8 μm2 in wild type compared with 4.6 μm2 in lqIs128[myr::unc-40]; P<0.01 (Fig. 2A)] with few filopodial protrusions, and exhibited a `treadmilling' behavior in which there was some movement around the edges of the growth cone but very little growth cone advance (see Movie 3 in the supplementary material). MYR::UNC-40 growth cones exhibited a decrease in filopodial formation rate (0.26 per minute in wild-type compared with 0.16 per minute in lqIs128[myr::unc-40]; P<0.01) (Fig. 2B). When filopodia did form, they had reduced duration (Fig. 2C) and reduced maximal length (Fig. 2D). These data suggest that UNC-40 normally has a role in inhibition of growth cone protrusion. In contrast to loss of function of unc-5 and unc-6(e78), myr::unc-40 also affected filopodia initiation rate. Thus, UNC-40 might also be involved in inhibition of filopodial initiation. Alternatively, filopodia might form at a normal rate in myr::unc-40 but are inhibited before they are visible in our imaging conditions, which might not be able to distinguish short or transient filopodia. Consistent with these defects in growth cone morphology, myr::unc-40 caused defects in VD and DD dorsal axon pathfinding, as determined by end-point analysis (55% defective axons in lqIs128[myr::unc-40]) (Fig. 1A). That loss of unc-5 and activation of myr::unc-40 had opposite effects on growth cone protrusiveness indicate that these are bona fide effects of the molecules on the growth cone and not due to a general perturbation of growth cone function.
Constitutively active MYR::UNC-40 requires UNC-5 to inhibit VD protrusion
We next tested whether the MYR::UNC-40-mediated reduction in growth cone protrusion was dependent on the presence of other netrin signaling components. We found that the MYR::UNC-40 phenotype was not dependent on UNC-6, as unc-6(ev400); myr::unc-40 double mutant VD growth cones resembled myr::unc-40 alone (Fig. 3A-D). This result is consistent with the idea that MYR::UNC-40 is an UNC-6-independent, constitutively active molecule. MYR::UNC-40 was also not dependent on endogenous UNC-40 (Fig. 3A-D). However, MYR::UNC-40 inhibition of protrusion was dependent upon UNC-5, as unc-5(e53) suppressed the effects of myr::unc-40 (Fig. 3A-F, see Movie 4 in the supplementary material). unc-5(e53) restored growth cone area, filopodia initiation rate, duration and maximal length (Fig. 3A-D). unc-5(e53); myr::unc-40 had a protrusion comparable with or greater than that of wild type, and in fact resembled the excessive protrusion seen in unc-5 mutants alone (e.g. filopodial duration was increased in unc-5(e53); lqIs128[myr::unc-40] compared with wild type; Fig. 3C).
Fig. 3.
MYR::UNC-40-mediated inhibition of VD growth cone protrusion requires UNC-5. (A-D) Quantification of filopodia dynamics in VD growth cones as described in Fig. 2. Error bars represent 2× standard error of the mean; asterisks indicate significant difference between lqIs128[myr::unc-40] and the double mutant (P<0.01) determined by two-sided t-test with unequal variance. (E,F) Fluorescent micrographs of typical mutant VD growth cones, with a line tracing of the growth cone perimeter and filopodial protrusions shown on the right. Scale bar: 5 μm.
These results suggest that MYR::UNC-40 requires UNC-5 to inhibit growth cone protrusion, consistent with previous results suggesting that an UNC-40/UNC-5 heterodimer mediates growth away from UNC-6/netrin. These results also suggest that formation of a putative MYR::UNC-40/UNC-5 heterodimer is independent of UNC-6/netrin, as would be expected of a ligand-independent constitutively active molecule.
Previous studies indicated that stationary or slow-moving growth cones often excess protrusion (Knobel et al., 1999), and we found that unc-5 mutant growth cones exhibited slow advance. Possibly, excess protrusion in unc-5 mutants was due in part to slow advance. We think this is unlikely because MYR::UNC-40 growth cones, which also displayed slow advance, had reduced protrusion; and because unc-5 loss of function suppressed the inhibition of protrusion of MYR::UNC-40 and restored protrusion to unc-5-like levels, indicating that UNC-5 was directly required for inhibiting protrusion.
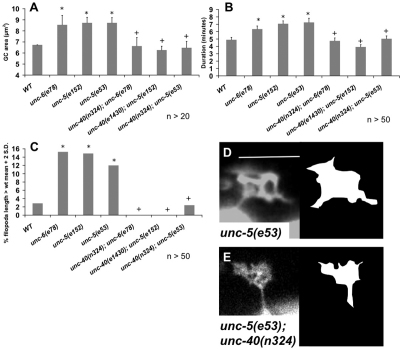
VD growth cone over-protrusive phenotypes of unc-5 and unc-6(e78) are suppressed by unc-40 mutation
unc-5 null and unc-6(e78) mutants but not unc-6(ev400) null mutants displayed increased growth cone protrusion, suggesting a role of UNC-6 in stimulating VD growth cone protrusion in the absence of UNC-5. We speculated that this might involve UNC-40. We constructed unc-5; unc-40 double mutants and found that they did not exhibit the over-protrusive phenotypes seen in the unc-5 single mutants (Fig. 4): growth cone size, filopodial duration and filopodia length (Fig. 4A-C) all returned to near-wild-type levels in the double mutants using two distinct null alleles of unc-40. Similarly, unc-40(n324) was required for the over-protrusive growth cones of unc-6(e78) mutants, as unc-6(e78); unc-40(n324) double mutants had near wild-type levels of protrusion (Fig. 4A-C). These results suggest that UNC-40 and UNC-6 have roles in stimulating VD growth cone protrusion in addition to roles in inhibition of protrusion along with UNC-5, as demonstrated by the MYR::UNC-40 results above. The above findings might also explain why unc-6-null mutants and unc-40 mutants display less severe growth cone protrusion defects than unc-5 mutants (i.e. UNC-6 and UNC-40 are required both to stimulate and to inhibit protrusion, and when they are gone an equilibrium between the two is maintained).
Fig. 4.
unc-5 mutant excessive growth cone protrusion requires UNC-40/DCC. (A-C) Quantification of filopodia dynamics in VD growth cones as described in Fig. 2. Error bars represent 2× standard error of the mean; asterisks indicate significant difference between wild type and the mutant (P<0.01); + indicates significant difference between double mutant and its respective protrusion-stimulating mutant (P<0.01) determined by two-sided t-test with unequal variance. (D,E) Fluorescent micrographs of typical mutant VD growth cones, with a line tracing of the growth cone perimeter and filopodial protrusions shown on the right. Scale bar: 5 μm.
This balance of protrusion in the growth cone leads to the idea that protrusion might be asymetrically affected across the growth cone, which might explain in part growth cone polarity and directed outgrowth. Indeed, in wild-type VD growth cones, dorsally directed filopodia had longer duration and length than ventrally directed filopodia, indicating that protrusion is greater in the direction furthest from the source of UNC-6/netrin (6.6 minutes dorsal versus 4.8 minutes ventral; P<0.02; n>50 filopodia; and 1.0 μm dorsal versus 0.8 μm ventral; P<0.02; n>50 filopodia).
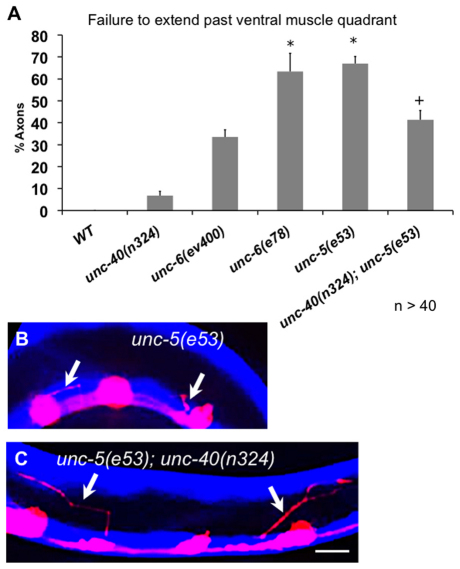
Axon pathfinding defects of unc-5 mutants are reduced by unc-40 mutation
Previous studies indicated that UNC-5 alone signaling mediates repulsion while close to the UNC-6/netrin source, whereas UNC-5 and UNC-40 signaling mediates repulsion at locations more distant from the UNC-6/netrin source (MacNeil et al., 2009). We labeled the ventral and dorsal muscle quadrants with CFP and the VD axons with mCherry, allowing us to quantify the number of axon trajectories that go awry before passing the ventral muscle quadrant, close to the normal source of UNC-6/netrin (Fig. 5) (Ishii et al., 1992). Indeed, most VD axon pathfinding defects in unc-40 mutants occurred after the axon passed the ventral muscle quadrant [>90% in unc-40(n324)] (Fig. 5A), whereas most unc-5(e53) null and unc-6(e78) defects occurred in or before the ventral muscle quadrant, with some axons apparently not emerging from the ventral nerve cord (Fig. 5A,B). unc-5(e53) and unc-6(e78) mutants also had significantly more severe defects than the unc-6(ev400) null, similar to the effects on growth cone protrusion. unc-40(n324); unc-5(e53) double mutants were less severe than unc-5(e53) alone, and had guidance defects of comparable severity to unc-6(ev400) alone. These data indicate that in unc-5 mutant VD growth cones, UNC-40/DCC may be mediating an attractive response to UNC-6/netrin, causing these axons to become attracted to, rather than repelled from, UNC-6/netrin, and thus misguided sooner than in the complete absence of UNC-6/netrin.
Fig. 5.
Severity of unc-5(e53) axon pathfinding defects is mitigated by loss of unc-40. (A) Percentage of VD axons that have pathfinding errors by the time they reach the dorsal edge of the ventral muscle quadrant. Error bars represent 2× standard error of the mean; asterisks indicate a significant difference between wild type and the mutant (P<0.01); + indicates a significant difference between unc-5(e53) alone and the unc-5(e53); unc-40(n324) double mutant (P<0.01) determined by two-sided t-test with unequal variance. (B,C) Representative images showing VD axons (arrows) with mCherry expression (red) after their complete outgrowth in L4 animals. Ventral and dorsal muscle quadrants with CFP expression are blue. In unc-5(e53) most axons do not advance beyond the ventral muscle quadrants, whereas in unc-5(e53); unc-40(n324), many axons extend past the ventral quadrants. Scale bar: 5 μm.
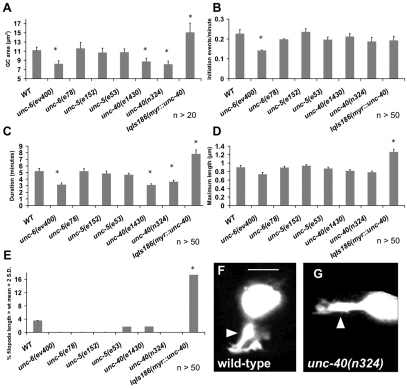
Netrin signaling component mutants modulate extent of protrusion of HSN growth cones
To study the roles of UNC-6 and UNC-40 in growth cones that are attracted to UNC-6/netrin, we turned to the HSN neurons. The bilateral HSN cell bodies reside laterally just behind the hermaphrodite vulva in the midsection of the animal. The HSN axons extend ventrally to the ventral nerve cord and then anteriorly to innervate the vulva. As previously reported, the axons of the HSN neurons require UNC-6 and UNC-40 for their ventralward migration (Fig. 1F-H) (Adler et al., 2006). In unc-6 and unc-40 mutants, the HSN axons often extend laterally instead of ventrally. The HSN displays a prominent growth cone at 18-19 hours post-hatching in the late L1 stage (see Movie 5 in the supplementary material). At this time, the growth cone resembles a thickened protrusion from the ventral region of the cell body with multiple filopodial protrusions, as previously reported (Adler et al., 2006). Over the next 12 hours into the mid L4 larval stage, the HSN growth cone remains dynamic and migrates ventrally to the ventral nerve cord. We analyzed HSN growth cones in time lapse at 19 hours post-hatching. At this time the wild-type HSN growth cone is on average 11.2 μm2 in size, with filopodia that form at an average rate of one every 4.4 minutes and endure for 5.2 minutes, with a maximal filopodial length of 0.9 μm (Fig. 6 and see Movie 5 in the supplementary material). The HSN growth cone is larger, but all dynamic filopodial characteristics are similar to those of the VD growth cones.
Fig. 6.
Netrin signaling mutants cause HSN growth cone morphology and dynamic defects. (A-E) Quantification of filopodia dynamics in HSN growth cones as described in Fig. 2. Error bars represent 2× standard error of the mean and asterisks indicate significant difference between wild type and the mutant (P<0.01) determined by two-sided t-test with unequal variance. (F) A typical wild-type HSN growth cone that protrudes ventrally from the cell body. (G) A typical unc-40(n324) HSN growth cone, which often protrudes anteriorly and laterally rather than ventrally from the cell body. Arrowheads indicate growth cones. Scale bar: 5 μm.
unc-40- and unc-6-null mutant HSN growth cones exhibited a decrease in protrusiveness (see Movie 6 in the supplementary material), with a decrease in growth cone, filopodial duration and filopodia length (Fig. 6A-D). unc-6(ev400)-null mutants but not unc-40 mutants also exhibited a decrease in filopodia formation rate (Fig. 6B). These results indicate that UNC-6/netrin and UNC-40/DCC are required for robust HSN growth cone protrusion. As expected, the unc-6(e78) mutation, which specifically attenuates UNC-5-mediated effects of UNC-6, had no effect on HSN growth cones (Fig. 6).
We drove the expression of MYR::UNC-40 in the HSNs using the unc-86 promoter. In contrast to inhibiting protrusion in the VDs, MYR::UNC-40 caused an increase in protrusiveness in the HSNs (see Movie 7 in the supplementary material), with an increase in growth cone size, filopodia duration and length (Fig. 6). This is the opposite effect seen with MYR::UNC-40 in the VDs, and suggests that in axons that do not express UNC-5, the MYR::UNC-40 molecules form an attractive complex that stimulates growth cone protrusion.
Considered with the results in the VD growth cones, these data suggest that UNC-6/netrin and it receptors UNC-40/DCC and UNC-5 control the extent of growth cone protrusion, including growth cone size and the maximal length and duration of growth cone filopodia. Inhibition versus stimulation of protrusion were correlated with repulsion versus attraction in response to UNC-6/netrin. However, in the VD growth cones, unc-6 mutants had only weak effects on growth cone protrusiveness but very strong VD axon guidance defects, indicating that regulation of growth cone protrusion is not the only role of UNC-6/netrin in growth cones during axon pathfinding.
UNC-5, UNC-6 and UNC-40 affect polarity of growth cone filopodia protrusion
Wild-type VD and HSN growth cones display highly polarized filopodial protrusion: in VD growth cones, most filopodia protrude dorsally away from UNC-6 (Fig. 7A,B,D); and in HSN growth cones, most filopodia protrude ventrally toward UNC-6 (Fig. 7E,F,H). We next determined whether orientation of filopodial protrusion was altered in unc-5, unc-6 and unc-40 mutants. Previous studies indicated that UNC-6 was required for polarized distribution of UNC-40 and MIG-10/lamellipodin on the ventral region of the HSN neuron and that unc-6 and unc-40 mutant HSN neurons displayed a loss of polarized ventral protrusion (Adler et al., 2006; Quinn et al., 2006; Quinn et al., 2008; Xu et al., 2009). To quantify polarity of filopodial protrusion, we divided the growth cones into four quadrants along the AP and DV axes, based upon the orientation of the animal, regardless of the direction that the growth cone was navigating (some VD growth cones in mutants were misguided and were migrating laterally or even ventrally; and many HSN growth cones protruded laterally rather than ventrally). In wild type, VD neurons growing along naked epidermis, the majority of filopodia (70%) extended from the dorsal half of the growth cone (Fig. 7A,B,D). unc-5- and unc-6-null mutations abolished this polarity such that filopodia protruded nearly equally in the dorsal and ventral directions (Fig. 7A,C,D). During the stage at which we imaged VD growth cones, unc-40-null mutations had no significant effect on dorsal ventral polarity of VD growth cone filopodial protrusion, nor did MYR::UNC-40. Anterior-posterior VD filopodial protrusion was not significantly different in wild-type or any mutant (Fig. 7D).
In wild-type HSN growth cones, the majority of filopodia extended from the ventral half of the growth cone, towards their target in the VNC (Fig. 7E,F,H). In HSN growth cones of all genotypes, there was a bias toward anterior versus posterior protrusion, as the HSN growth cone generally extends ventrally from the anterior of the cell body. Loss of UNC-5 did not affect dorsal-ventral polarity, which is consistent with the observation that UNC-5 is not expressed in the HSN (Fig. 7E,H). However, loss of UNC-40/DCC or UNC-6/netrin abolished the ventral polarity of filopodia extension (Fig. 7E,G,H), and the growth cone often extended anterolaterally instead of ventrally (Fig. 7G). These results are consistent with previous results showing that UNC-6 localizes UNC-40 to the ventral surface of the HSN and, in an unc-6 mutant, UNC-40 localizes uniformly around the cell body and protrusions extend from around the cell body (Xu et al., 2009). These results indicate that UNC-5, UNC-6 and UNC-40 control the polarity of filopodial protrusion from growth cones that are repelled (VDs) or attracted (HSNs) to UNC-6/netrin.
UNC-5 and UNC-6 control polarity of growth cone filamentous actin
Growth cone protrusion, including the lamellipodial growth cone body and filopodial extensions, involves dynamic regulation of the actin cytoskeleton (Quinn and Wadsworth, 2008). In the VDs and the PQR dendrite growth cones, the actin regulators Arp2/3, UNC-115/abLIM and UNC-34/enabled are required for robust filopodial extensions (Norris et al., 2009). We assayed filamentous actin (F-actin) in the VD growth cones, using a construct in which the F-actin binding domain of the spectraplakin VAB-10 is fused to GFP that has been used to monitor F-actin in other cells in living C. elegans (Bosher et al., 2003; Patel et al., 2008). In wild-type VD growth cones, this VAB-10ABD::GFP construct localized preferentially to the dorsal leading edge of the lamellipodia and in filopodial protrusions (Fig. 8A,B,E). On average, VAB-10ABD::GFP showed 1.22-fold more accumulation in the dorsal half of growth cones compared with the ventral halves (Fig. 8G). In unc-6(ev400)-null mutants and unc-5(e53)-null mutants, VAB-10ABD::GFP no longer showed a preferential dorsal leading edge accumulation (Fig. 8C,D,G). Rather, VAB-10ABD::GFP distribution was randomized across the growth cone (Fig. 8E) and in some growth cones showed a ventral bias (Fig. 8C). In unc-6(ev400), F-actin was often seen to accumulate at both the dorsal and ventral margins of the growth cone (Fig. 8F). F-actin accumulated in filopodial protrusions at the margin, so this might represent randomized filopodial protrusion, as seen in the growth cone in Fig. 8E. No obvious differences in overall levels of F-actin were detected, but slight changes would not be distinguished using this assay. unc-40-null mutations had no effect on VAB-10ABD::GFP distribution. These results suggest UNC-5 and UNC-6 control the distribution of F-actin in the VD growth cone away from the UNC-6/netrin source and towards the protrusive dorsal edge of the growth cones.
DISCUSSION
UNC-6/netrin and its receptors modulate extent of growth cone protrusion
We show here that in UNC-6-repelled VD neurons, which express both UNC-40/DCC and UNC-5, loss of unc-5 causes an increase in protrusive activity. We show that this is probably due to an increase in protrusion-stimulating activity of UNC-6/netrin and UNC-40/DCC signaling in the absence of protrusion-limiting UNC-5. In the HSNs, we also show that loss of UNC-6/netrin or UNC-40/DCC causes a reduction in the extent of growth cone protrusion, probably owing to a loss of attractive signaling. Our results suggest that UNC-6/netrin mediates a balance of protrusive activity in the growth cone, with UNC-40 driving protrusion and UNC-5/UNC-40 inhibiting protrusion. This might set up a gradient of protrusive activity, which in the VD growth cone results in more protrusion dorsally and less ventrally (see Movie 1 in the supplementary material). This mechanism might in part explain growth cone polarity and directed outgrowth during growth cone pathfinding. The fact that there is still a low level of dorsoventral guidance in unc-6 mutants suggests that other guidance signals, such as UNC-129 or SLT-1 may still be affecting the migration of VD and HSN growth cones (e.g. SLT-1 preventing HSN axons from migrating dorsally).
Relationship between growth cone attraction and protrusion
Our findings suggest that there is a link between an attractive cue and stimulation of protrusion, and between a repulsive cue and inhibition of protrusion in the growth cone. When the repulsive receptor UNC-5 is removed in VDs, the result is an overly protrusive phenotype. When the attractive UNC-40/DCC receptor, or its ligand UNC-6/netrin, are removed in HSNs, the result is reduced protrusiveness.
Similarly, when an activated version of the attractive receptor UNC-40/DCC is expressed in HSNs, it causes an increase in protrusiveness. However, when it is expressed in VDs containing UNC-5, it causes excess inhibition of protrusion, probably through the formation of repulsive protrusion-limiting UNC-5/UNC-40/DCC heterodimers.
These findings suggest a broader theme in which the sensation of an attractive cue causes stimulation of protrusion, whereas a repulsive cue causes inhibition of protrusion. These in vivo morphological results are also supported by previous in vitro studies (Shekarabi and Kennedy, 2002; Shekarabi et al., 2005).
UNC-6/netrin and its receptors affect polarity of protrusion
Previous studies have shown that UNC-6/netrin is involved in establishment of initial protrusion polarity in the growth cone (Adler et al., 2006). We expand upon these findings and show that UNC-6/netrin, UNC-40/DCC and UNC-5 are all involved in controlling the polarity of protrusion in the growth cone.
We show here that UNC-6/netrin is required for appropriately polarizing the protrusions of VD and HSN growth cones. In unc-6 mutants, both VD and HSN growth cones lose filopodia and F-actin polarization. We also show that in VDs, UNC-5 is required for appropriate polarization of filopodia and F-actin, and that in the HSNs UNC-40/DCC is required for appropriate polarization of filopodia.
Guidance in response to UNC-6/netrin might involve a balance of protrusion and inhibition of protrusion
We have shown that UNC-6/netrin signaling affects both the extent of protrusion and polarity of protrusion and F-actin accumulation. Possibly, polarity of protrusion is achieved by asymmetric inhibition and stimulation of protrusion across the growth cone (e.g. in VD growth cones, ventral protrusion is more significantly inhibited, resulting in net dorsal protrusion) (see Movie 1 in the supplementary material). Consistent with this idea, we found that the over-protrusive effects of unc-5 and unc-6(e78) in VD growth cones required functional UNC-40, indicating that even in growth cones repelled from UNC-6/netrin there is still a latent pro-protrusive response to UNC-6/netrin. This is in agreement with the observation that there was a decrease in filopodia length in unc-40 VDs. Possibly, growth cone polarity involves balancing stimulation versus inhibition of protrusion across the growth cone in response to UNC-6/netrin. Indeed, we found a slight but significant increase in the duration and length of filopodia in the dorsal region of the VD growth cone compared with the ventral region. Although this effect was small, the outcome on growth cone movement when integrated over time could be significant. An attractive model is that UNC-40 signaling alone generally stimulates protrusion in response to UNC-6/netrin, and that UNC-5 and UNC-40 signaling inhibits protrusion in response to UNC-6/netrin close to the ventral UNC-6/netrin source (see Fig. S1 in the supplementary material). Growth cone polarity and dorsal migration might depend upon asymmetric distribution of protrusive activities. Although the mechanisms are unclear, such asymmetry might be achieved via differential receptor complex sensitivity to UNC-6, or by feedback that consolidates asymmetry (e.g. receptor trafficking). Supporting the latter notion, localization of UNC-40 and the SLT-1/Slit receptor SAX-3 by the UNC-73/Trio GEF, MIG-2,/RhoG and VAB-8 are important for receptor function (Levy-Strumpf and Culotti, 2007; Watari-Goshima et al., 2007).
Endpoint analysis of VD axons also indicates that pathfinding might involve a balance of protrusion and inhibition of protrusion. unc-5 and unc-6(e78) mutants had more severe axon pathfinding errors than a null unc-6 allele, but removing unc-40 in either one of these backgrounds reverted the phenotype severity to that of the unc-6 null. This indicates that excess protrusion may lead directly to increased axon pathfinding defect severity. Our results also suggest an additional mechanism for the previously observed high level of axon pathfinding defects at short distances from the UNC-6/netrin source in unc-5 mutants (MacNeil et al., 2009). These authors postulated that UNC-5 homodimers mediated repulsion near the UNC-6/netrin source, and that UNC-5/UNC-40 heterodimers mediated repulsion further from the UNC-6/netrin source loss. Our results suggest that in unc-5 mutants, latent UNC-40 mediated attraction to UNC-6/netrin steers the growth cone back toward the ventral source of UNC-6, resulting in severe axon pathfinding defects. These two mechanisms are not mutually exclusive and both might contribute to the severe axon pathfinding defects of unc-5 mutants compared with unc-40 and unc-6 mutants.
UNC-6/netrin signaling controls growth cone filopodial dynamics
In contrast to involving balanced protrusion across the growth cone, the extent of protrusion and the polarity of protrusion might be regulated by separate mechanisms. Supporting this idea are our observations that in unc-5 and unc-6(e78) mutants, the length and duration of VD growth cone filopodia are increased, including dorsally directed filopodia, indicating an effect on filopodial protrusion independent of their initiation or polarity. Furthermore, MYR::UNC-40 activity in the HSN affects extent of protrusion but has little effect on polarity of protrusion. Indeed, some filopodia in unc-5 mutants grow very long and persist throughout the time of imaging. Possibly, these persistent filopodia are the basis of the ectopic axon branches observed in endpoint analysis of unc-5 and unc-6 mutants, and that require functional UNC-40.
In summary, our results suggest UNC-6/netrin controls a balance of protrusion within a growth cone, with UNC-40 driving protrusion and UNC-5/UNC-40 inhibiting protrusion. Our results also suggest that these forces might be asymmetric in the growth cone, resulting in growth cone polarity and directed growth cone protrusion and advance.
Supplementary Material
Acknowledgments
The authors thank members of the Lundquist and Ackley labs for helpful discussion, S. Hamdeh for creation of numerous strains, E. Struckhoff for technical assistance, M. Tourtellot for the Spoke12 program, and B. Ackley (Lawrence, KS, USA), Y. Jin (San Diego, CA, USA), A. Chisholm (San Diego, CA, USA), and M. Soto (Piscataway, NJ, USA) for reagents. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR).
Footnotes
Funding
This work was supported by NIH grant NS40945 to E.A.L. and by NIH grant P20 RR016475 from the INBRE/IDEA Program of the National Center for Research Resources (J. Hunt is the P.I.). Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.068841/-/DC1
References
- Adler C. E., Fetter R. D., Bargmann C. I. (2006). UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation. Nat. Neurosci. 9, 511-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosher J. M., Hahn B. S., Legouis R., Sookhareea S., Weimer R. M., Gansmuller A., Chisholm A. D., Rose A. M., Bessereau J. L, Labouesse M. (2003). The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 161, 757-768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. S., Zheng H., Su M. W., Wilk R., Killeen M. T., Hedgecock E. M., Culotti J. G. (1996). UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell 87, 187-195 [DOI] [PubMed] [Google Scholar]
- Gallo G., Letourneau P. C. (2004). Regulation of growth cone actin filaments by guidance cues. J. Neurobiol. 58, 92-102 [DOI] [PubMed] [Google Scholar]
- Gitai Z., Yu T. W., Lundquist E. A., Tessier-Lavigne M., Bargmann C. I. (2003). The netrin receptor UNC-40/DCC stimulates axon attraction and outgrowth through enabled and, in parallel, Rac and UNC-115/AbLIM. Neuron 37, 53-65 [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H. (1990). The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4, 61-85 [DOI] [PubMed] [Google Scholar]
- Hong K., Hinck L., Nishiyama M., Poo M. M., Tessier-Lavigne M., Stein E. (1999). A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 97, 927-941 [DOI] [PubMed] [Google Scholar]
- Ishii N., Wadsworth W. G., Stern B. D., Culotti J. G., Hedgecock E. M. (1992). UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 9, 873-881 [DOI] [PubMed] [Google Scholar]
- Jin Y., Jorgensen E., Hartwieg E., Horvitz H. R. (1999). The Caenorhabditis elegans gene unc-25 encodes glutamic acid decarboxylase and is required for synaptic transmission but not synaptic development. J. Neurosci. 19, 539-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobel K. M., Jorgensen E. M., Bastiani M. J. (1999). Growth cones stall and collapse during axon outgrowth in Caenorhabditis elegans. Development 126, 4489-4498 [DOI] [PubMed] [Google Scholar]
- Leonardo E. D., Hinck L., Masu M., Keino-Masu K., Ackerman S. L., Tessier-Lavigne M. (1997). Vertebrate homologs of C. elegans UNC-5 are candidate netrin receptors. Nature 386, 833-838 [DOI] [PubMed] [Google Scholar]
- Levy-Strumpf N., Culotti J. G. (2007). VAB-8, UNC-73 and MIG-2 regulate axon polarity and cell migration functions of UNC-40 in C. elegans. Nat. Neurosci. 10, 161-168 [DOI] [PubMed] [Google Scholar]
- Lim Y. S., Wadsworth W. G. (2002). Identification of domains of netrin UNC-6 that mediate attractive and repulsive guidance and responses from cells and growth cones. J. Neurosci. 22, 7080-7087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil L. T., Hardy W. R., Pawson T., Wrana J. L., Culotti J. G. (2009). UNC-129 regulates the balance between UNC-40 dependent and independent UNC-5 signaling pathways. Nat. Neurosci. 12, 150-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C., Fire A. (1995). DNA transformation. Methods Cell Biol. 48, 451-482 [PubMed] [Google Scholar]
- Montell D. J. (1999). The genetics of cell migration in Drosophila melanogaster and Caenorhabditis elegans development. Development 126, 3035-3046 [DOI] [PubMed] [Google Scholar]
- Moore S. W., Tessier-Lavigne M., Kennedy T. E. (2007). Netrins and their receptors. Adv. Exp. Med. Biol. 621, 17-31 [DOI] [PubMed] [Google Scholar]
- Mortimer D., Fothergill T., Pujic Z., Richards L. J., Goodhill G. J. (2008). Growth cone chemotaxis. Trends Neurosci. 31, 90-98 [DOI] [PubMed] [Google Scholar]
- Norris A. D., Dyer J. O., Lundquist E. A. (2009). The Arp2/3 complex, UNC-115/abLIM, and UNC-34/Enabled regulate axon guidance and growth cone filopodia formation in Caenorhabditis elegans. Neural Dev. 4, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C. W., Flynn K. C., Bamburg J. R. (2008). Actin-binding proteins take the reins in growth cones. Nat. Rev. Neurosci. 9, 136-147 [DOI] [PubMed] [Google Scholar]
- Pan Y., Liu G., Fang M., Shen L., Wang L., Han Y., Shan D., Wang X. (2010). Abnormal expression of netrin-G2 in temporal lobe epilepsy neurons in humans and a rat model. Exp. Neurol. 224, 340-346 [DOI] [PubMed] [Google Scholar]
- Patel F. B., Bernadskaya Y. Y., Chen E., Jobanputra A., Pooladi Z., Freeman K. L., Gally C., Mohler W. A., Soto M. C. (2008). The WAVE/SCAR complex promotes polarized cell movements and actin enrichment in epithelia during C. elegans embryogenesis. Dev. Biol. 324, 297-309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C. C., Wadsworth W. G. (2008). Axon guidance: asymmetric signaling orients polarized outgrowth. Trends Cell Biol. 18, 597-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn C. C., Pfeil D. S., Chen E., Stovall E. L., Harden M. V., Gavin M. K., Forrester W. C., Ryder E. F., Soto M. C., Wadsworth W. G. (2006). UNC-6/netrin and SLT-1/slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/lamellipodin. Curr. Biol. 16, 845-853 [DOI] [PubMed] [Google Scholar]
- Quinn C. C., Pfeil D. S., Wadsworth W. G. (2008). CED-10/Rac1 mediates axon guidance by regulating the asymmetric distribution of MIG-10/lamellipodin. Curr. Biol. 18, 808-813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekarabi M., Kennedy T. E. (2002). The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1. Mol. Cell. Neurosci. 19, 1-17 [DOI] [PubMed] [Google Scholar]
- Shekarabi M., Moore S. W., Tritsch N. X., Morris S. J., Bouchard J. F., Kennedy T. E. (2005). Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J. Neurosci. 25, 3132-3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K., Bargmann C. I. (2003). The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell 112, 619-630 [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M., Goodman C. S. (1996). The molecular biology of axon guidance. Science 274, 1123-1133 [DOI] [PubMed] [Google Scholar]
- Watari-Goshima N., Ogura K., Wolf F. W., Goshima Y., Garriga G. (2007). C. elegans VAB-8 and UNC-73 regulate the SAX-3 receptor to direct cell and growth-cone migrations. Nat. Neurosci. 10, 169-176 [DOI] [PubMed] [Google Scholar]
- Weinkove D., Bastiani M., Chessa T. A., Joshi D., Hauth L., Cooke F. T., Divecha N., Schuske K. (2008). Overexpression of PPK-1, the Caenorhabditis elegans Type I PIP kinase, inhibits growth cone collapse in the developing nervous system and causes axonal degeneration in adults. Dev. Biol. 313, 384-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Li H., Wadsworth W. G. (2009). The roles of multiple UNC-40 (DCC) receptor-mediated signals in determining neuronal asymmetry induced by the UNC-6 (netrin) ligand. Genetics 183, 941-949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F. Q., Cohan C. S. (2004). How actin filaments and microtubules steer growth cones to their targets. J. Neurobiol. 58, 84-91 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.