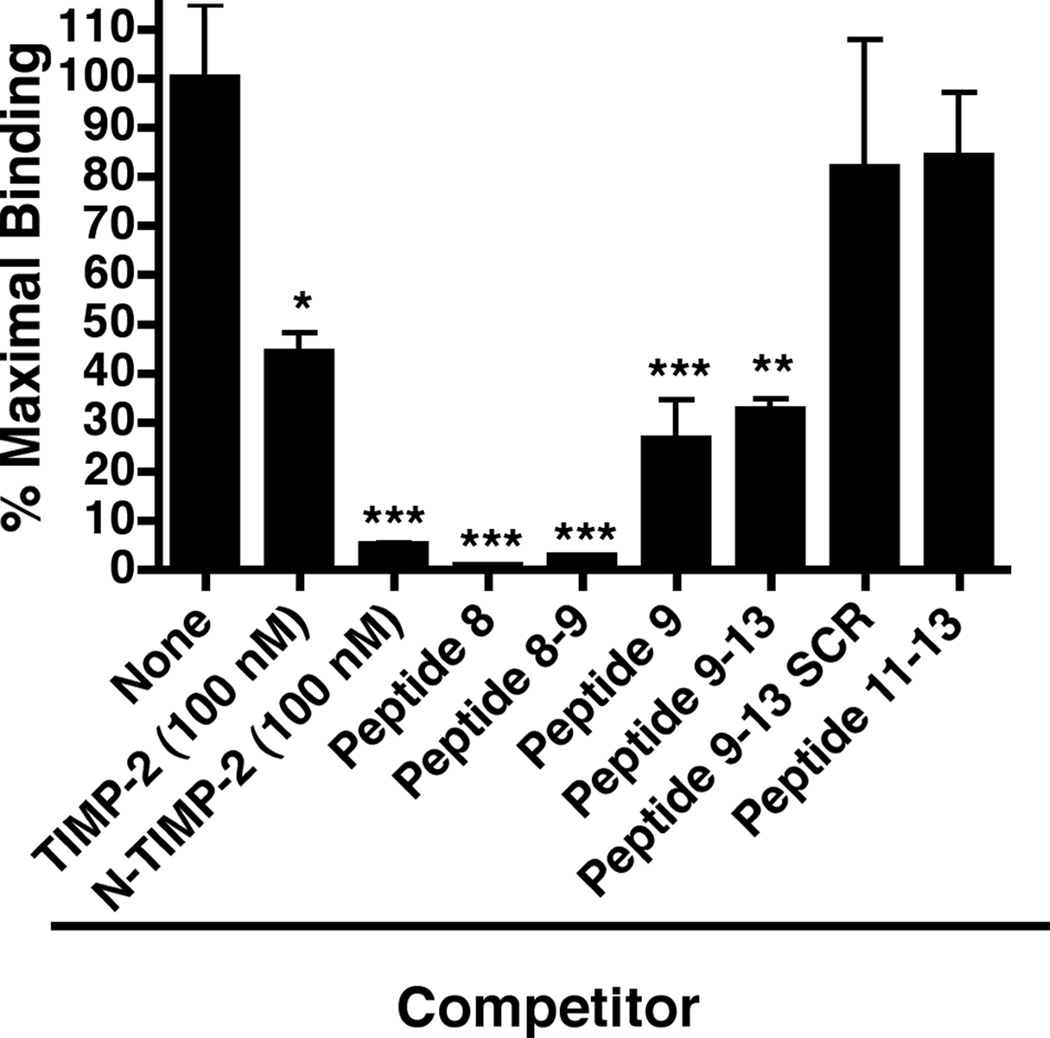
Figure 3.
Peptide competition experiments for α3β1-binding. Wells were coated with Ala+TIMP-2 and blocked as descried in Materials and methods. The α3β1-binding activity was then assayed in the absence (maximal binding) or the presence of TIMP-2 (100 nM, * p<0.01), N-TIMP-2 (100 nM, *** p<0.0005), or synthetic peptides (all added at 1 µM final concentration). The results demonstrate that peptide 8, peptide 9, as well as the overlap region between these two peptides, peptide 8–9 significantly (*** p<0.0005) compete for the ability of α3β1-binding to the Ala+TIMP-2 coating (see Supplemental Data Table 2 for specific peptide sequences). In addition, the results confirm the importance of the C-terminal portion of peptide 9 in α3β1-binding, in that peptide 9–13 retains significant (** p<0.005) competitive activity, whereas loss of peptide 9 sequence as in peptide 11–13 or disruption of the linear peptide 9 sequence (peptide 9–13 SCR) resulted in substantial loss of competitive binding activity. The results confirm the findings of the peptide array analysis of TIMP-2 peptides.