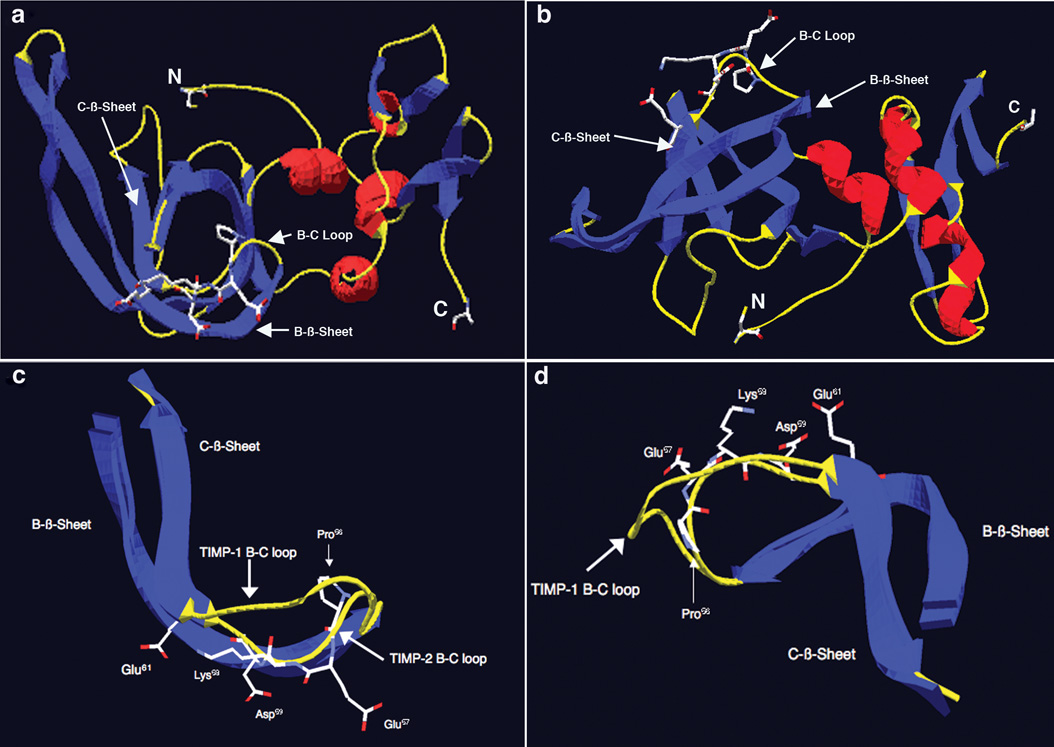
Figure 6.
Structure analysis and identification of potentially important amino acid residues for the α3β1-binding activity of TIMP-2 localized to the B-C loop. a) Structure of TIMP-2 viewed perpendicular to the long axis of the N-terminal OB fold. Blue denotes β-sheets, red denotes α-helices and yellow denotes loop structures. N is the amino terminal cysteine and C is the carboxyl terminal alanine residue. Also shown is the surface localization of the charged residues of the 23 amino acid α3β1-binding region of the B-C loop. b) Structure of TIMP-2 viewed from below, all other designations are the same as in A. c) B-C loops of TIMP-2 and TIMP-1 overlaid and viewed perpendicular to the long axis of the OB fold, demonstrating the kink induced by Pro56 that results in projection of the charged amino acid residues Glu57, Lys58, Asp59 and Glu61 of the 24 amino acid α3β1-binding domain away from the interior of the OB fold towards the surface of the TIMP-2 molecule. d) B-C loops of TIMP-2 and TIMP-1 viewed from above again showing projection of amino acid residues Glu57, Lys58, Asp59 and Glu61 towards the surface and away from the interior of the TIMP-2 molecule.