Abstract
Background and Aims
Genetic characterization and phylogenetic analysis of the oldest trees could be a powerful tool both for germplasm collection and for understanding the earliest origins of clonally propagated fruit crops. The olive tree (Olea europaea L.) is a suitable model to study the origin of cultivars due to its long lifespan, resulting in the existence of both centennial and millennial trees across the Mediterranean Basin.
Methods
The genetic identity and diversity as well as the phylogenetic relationships among the oldest wild and cultivated olives of southern Spain were evaluated by analysing simple sequence repeat markers. Samples from both the canopy and the roots of each tree were analysed to distinguish which trees were self-rooted and which were grafted. The ancient olives were also put into chronological order to infer the antiquity of traditional olive cultivars.
Key Results
Only 9·6 % out of 104 a priori cultivated ancient genotypes matched current olive cultivars. The percentage of unidentified genotypes was higher among the oldest olives, which could be because they belong to ancient unknown cultivars or because of possible intra-cultivar variability. Comparing the observed patterns of genetic variation made it possible to distinguish which trees were grafted onto putative wild olives.
Conclusions
This study of ancient olives has been fruitful both for germplasm collection and for enlarging our knowledge about olive domestication. The findings suggest that grafting pre-existing wild olives with olive cultivars was linked to the beginnings of olive growing. Additionally, the low number of genotypes identified in current cultivars points out that the ancient olives from southern Spain constitute a priceless reservoir of genetic diversity.
Keywords: Olea europaea, wild olives, traditional cultivars, microsatellite markers, intracultivar variability, domestication, in situ conservation
INTRODUCTION
The genetic characterization and phylogenetic analysis of the oldest trees could be a powerful tool both for increasing germplasm collection and for understanding the earliest origins of clonally propagated fruit crops. These trees might be unknown primitive cultivars and their study might provide key clues to determine how fruit crops have been domesticated (Harris et al., 2002).
The olive tree (Olea europaea) is a suitable model to perform such studies due to its long lifespan, resulting in the presence of both centennial and millennial trees across the Mediterranean Basin. Previous studies have genetically characterized a low number of ancient olives and only a small proportion of these trees matched current olive cultivars (Baldoni et al., 2006; Erre et al., 2010). These studies supported the hypothesis that ancient olive trees might be unknown traditional cultivars that remained uncharacterized and suggested they might represent early stages in the domestication processes of the olive.
Consequently, the characterization and conservation of the ancient olive germplasm is a priority task because these trees are progressively disappearing due to their increasing ornamental value and to the progressive transformation of traditional olive groves into new commercial orchards (Muñoz-Diez, 2008; Rallo and Muñoz-Díez, 2010). Additionally, the outstanding performance of ancient olives through time makes their agronomical evaluation especially interesting for breeding purposes.
The study of the genetic diversity of ancient olives may also be helpful in understanding olive domestication. This approach is particularly useful with the olive due to the lack of information about cultivar pedigrees and the difficulties in amplifying fossil DNA from olives due to degradation (Elbaum et al., 2006; Hansson and Foley, 2008). The olive was probably domesticated in the Middle East about 6000 years ago (Zohary and Spiegel-Roy, 1975). Afterwards, commercial shipping spread this crop westward across the Mediterranean Basin. Nevertheless, determining the origin of olive cultivars is still a complex and unresolved task. The existence of several multilocal domestication events has been proposed based on molecular studies (Claros et al., 2000; Besnard et al., 2001). This hypothesis is also supported by two main lines of evidence: (1) the huge diversity of different clonally propagated cultivars found in all of the traditional olive-producing countries (Rallo, 2005) and (2) the presence of the wild olive (Olea europaea subsp. europaea var. sylvestris), the ancestor of the cultivated olive, as indigenous vegetation throughout the humid and sub-humid thermo-Mediterranean areas. However, a weak association between olive cultivars and their putative areas of origin has also been reported (Angiolillo et al., 1999; Nikoloudakis et al., 2003; Hagidimitriou et al., 2005; Montemurro et al., 2005; Baldoni et al., 2006; Belaj et al., 2010). Continuous crossings among cultivars and autochthonous material (wild or cultivated), along with subsequent selection cycles and clonal propagation, have probably blurred genetic patterns on olive cultivars. However, these patterns could still be detectable in the earliest cultivars, as represented by the oldest trees.
The Andalusia region of southern Spain is the main olive-oil producer in the world (International Olive Council, 2010) and is a good area to test our hypothesis for three main reasons: (1) wild olive was already present and used by man since Neolithic times (Terral, 2000; Rodriguez-Ariza and Moya, 2005); (2) a rich diversity of traditional cultivars have been systematically surveyed and characterized by morphological descriptors and molecular markers (Barranco and Rallo, 2000; Barranco et al., 2005); (3) these cultivars are likely to be the product of local selection processes because the introduction of olive genetic pools from abroad has been limited in the whole West Mediterranean Basin (Besnard et al., 2001; Baldoni et al., 2009). Moreover, southern Spain, contains centennial and millennial olives as well as large and genuine wild olive forests in undisturbed areas that may be considered hot spots of olive genetic diversity (Muñoz-Diez et al., 2004; Rubio de Casas et al., 2006; Belaj et al., 2007, 2010; Muñoz-Diez, 2008).
This study presents the results of the first extensive sampling and systematic genetic analysis of ancient olive trees in Spain. Southern Spain was surveyed for ancient olives which were genetically characterized by simple sequence repeat (SSR) markers with the main goal of enhancing our knowledge about their cultivar identity, genetic variability and phylogenetic relationships. These ancient olives were also put into chronological order hoping to infer the antiquity of traditional olive cultivars. The findings demonstrate that Andalusian ancient olives constitute an unexploited reservoir of genetic diversity that can be considered a priceless in situ germplasm collection of genetic resources.
MATERIALS AND METHODS
Plant material
One hundred and sixty (29 wild and 131 cultivated) ancient olive trees (Olea europaea L.) were localized and sampled after a systematic survey of the Andalusian region (Fig. 1 and Table 1). The regional government as well as olive producers participated along with the Agronomy Department of the University of Cordoba in the localization of the most ancient olives of each province of Andalusia. This region is divided into eight provinces that can be grouped into three main geographical areas: Eastern (Almeria, Granada, Jaen), Central (Cordoba and Malaga) and Western (Cadiz, Huelva and Sevilla).
Fig. 1.
Four ancient olives sampled in this study. The majority of the cultivated and wild olives had only one trunk (A, B, respectively); however, 36 cultivated olives had more than one trunk as a result of traditional propagation techniques by hard-cuttings (C, D).
Table 1.
Number of ancient olives trees (wild and cultivated) included in this study, their number of trunks from the ground and the number of samples analysed, taking into account that both the canopy and the suckers of the base of each trunk were sampled
| Number of trunks |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Total | |
| Number of trees | 124 | 20 | 13 | 2 | 1 | 160 |
| Canopy samples | 124 | 40 | 39 | 8 | 5 | 216 |
| Trunk samples | 123 | 40 | 39 | 8 | 5 | 215 |
| Total samples | 247 | 80 | 78 | 16 | 10 | 431 |
The cultivated status of the trees was inferred by taking into account their location in olive orchards, pruning architecture and interviews with the owners. To distinguish between self-rooted and grafted trees, both the root and the canopy of all the trees were sampled. Therefore, leaves from the canopy and from sprouts rising up from the base of each tree trunk were collected. In total, 431 samples were analysed taking into account that 36 trees were composed of more than one trunk (Table 1 and Fig. 1C, D).
PCR reactions and SSR analysis
Total DNA was extracted from young olive leaves according to the protocol described by de la Rosa et al. (2002). Genetic characterization of these samples was carried out by amplifying 14 SSR markers (Supplementary Data Table S1, available online) according to the PCR conditions described by de la Rosa et al. (2002). These makers have been used to identify the WOGB (World Olive Germplasm Bank, located in the IFAPA research centre in Cordoba, Spain) and were selected for their high resolution in previous studies (Bracci et al., 2009; Belaj et al., 2010; Erre et al., 2010).
Ancient olives: identification and genetic relationships
As a preliminary step, the genotypes from both the canopy and the base of each trunk were compared to discard duplicated genotypes. Only the different SSR profiles per tree were included for further study. Genetic diversity parameters for each microsatellite locus in the samples were as follows: average number of alleles (Na), observed heterozygosity (Ho), expected heterozygosity (He), inbreeding coefficient (F) and polymorphism information content (PIC; Botstein et al., 1980). These parameters were calculated using the PowerMarker V3·23 (Liu and Muse, 2005) software package. The probability of identity (IP; Paetkau and Strobeck, 1994) for each locus and for the whole SSR set (accumulated IP) was calculated by the Gimlet software v1·3.3 (Valiere, 2002). Null allele frequency per locus (An) was tested using MICRO-CHECKER 2·2 (van Oosterhout et al., 2006).
To identify the ancient genotypes, their SSR profiles were compared with the WOGB SSR profiles, which currently include the genotypes of >350 olive cultivars of 21 countries (I. Trujillo, University of Cordoba, Spain, unpubl. res.). These data broadly represent the genetic diversity of the Spanish and specifically Andalusian olive cultivars because they include the profiles of 219 cultivars from the whole country, 120 of them being from the southern area. Wild-olive profiles were also compared with those obtained in previous studies that were focused on wild-olive diversity in Spain and included the SSR profiles of 239 wild olives collected around the whole country (Muñoz-Diez, 2008; Belaj et al., 2007, 2010). A matrix was built up with the different SSR profiles that scored the amplified alleles as present or absent to evaluate the genetic relationships between ancient olive samples. This matrix was used to perform a cluster analysis based on the unweighted pair group method with arithmetic mean (UPGMA) algorithm using Dice's similarity index (Dice, 1945) implemented in the statistical software NTSYS-PC v2·02 (Rohlf, 1998). The correlation coefficient between the similarity matrix and the cophenetic values matrix was computed to test the goodness-of-fit for the cluster analysis.
Population structure of ancient olives
BAPS (Corander and Marttinen, 2006; Corander et al., 2008) and Structure version 2·3 (Pritchard et al., 2000) were used to perform a Bayesian analysis to identify hidden population structure with no a priori grouping assumptions, by clustering individuals into genetically distinguishable groups on the basis of allele frequencies and linkage disequilibrium (LD). BAPS and Structure differ in their approach to estimating admixture. Whereas BAPS first infers the most likely individual clusters in the sample population and then performs the most likely admixture of genotypes (Corander et al., 2003), Structure infers the highest likelihood of both the individual clusters and the admixture of genotypes using allele frequency and LD information from the dataset directly. K values in both programs were set ranging from 1 to 24 (number of possible different groups).
The admixture coefficients for the individuals in BAPS were estimated using 1000 iterations, 200 reference individuals/population and 100 reference individuals. Simulations were repeated ten times for each K value, and the resulting matrices of estimated cluster membership coefficients were permuted with CLUMPP (Jakobsson and Rosenberg, 2007) to account for differences among the runs.
Individual and admixture analyses were performed using the Structure software with the following assumptions: (a) 100 000 generations of ‘burn-in’ and 100 000 Markov chain Monte Carlo (MCMC) generations were used for each value of K; and (b) individuals were assumed to have a mixed ancestry, with correlated allele frequencies among populations. Simulations were repeated ten times for each value of K, and the resulting matrices of estimated cluster membership coefficients were also permuted with CLUMPP. The final matrix for each K value was visualized with DISTRUCT (Rosenberg, 2004). The optimal number of genetic clusters was determined using the ad hoc statistic ΔK, based on the rate of change in the log probability of data between successive K values (Evanno et al., 2005).
Antiquity of cultivars
Several studies have indicated a strong relationship between trunk diameter and tree age (Rozas, 2003, 2004), although considerable intraspecific variation may exist within trees of the same age due to differences in site characteristics (Burley et al., 2007). Ancient olive trees are in the senescent phase and usually have a fragile hollow stem, making the use of destructive sampling (cores or cross-sections) impossible. In this situation, a size–age relationship appears to be the most suitable method to arrange approximately ancient olives according to their age. The diameter of 135 ancient olives was measured at 1 m from the ground and was used to establish an approximate chronological ranking of these trees. Trees with seriously damaged trunks were excluded from this analysis (Table 2). The diameter of the ancient olives ranged between 0·62 and 2·72 m and was used to classify the trees into four categories according to their trunk diameter (d): first, d ≥ 2 m; second, 2 m > d ≥ 1·5 m; third, 1·5 m > d ≥ 1·0 m; and fourth, d < 1·0 m.
Table 2.
Classification of 135 ancient olive trees according to their trunk diameter
| Diameter, d (m) | Wild | Cultivated | Grafted (%) | Identified (%) | Cultivars |
|---|---|---|---|---|---|
| d ≥ 2 | 4 | 13 | 8 (61·5) | 4 (30·8) | ‘Lechin de Granada’, ‘Gordal Sevillana’ |
| 2 > d ≥ 1·5 | 3 | 28 | 19 (67·8) | 6 (21·4) | ‘Gordal Sevillana’, ‘Lechin de Granada’, ‘Verdial de Velez Malaga’ |
| 1·5 > d ≥ 1·0 | 10 | 35 | 16 (45·7) | 12 (34·3) | ‘Lechin de Granada’, ‘Lechin de Sevilla’, ‘Royal de Cazorla’, ‘Verdial de Huevar’, ‘Verdial de Velez-Malaga’ |
The number and percentage of grafted and identified trees are specified for cultivated trees as well as the cultivars identified in each category
RESULTS
Overall genetic diversity
The analysis of the SSR profiles led to the discrimination of 134 different genotypes (104 cultivated and 30 wild) among the 160 ancient olive trees included in this study. The SSR profiles from the canopy and from the root suckers around the base of the trunk did not match in 53 olives (52 cultivated and one wild). In all these cases both profiles (canopy and base of the trunk) showed extensive allelic differences, indicating that these trees were grafted and therefore composed by two genotypes: rootstock and grafted cultivar. Additionally, in ‘multi-trunk’ olives, the samples from the canopy shared the same genotype as well as the samples from the base of each trunk in the case of grafted ‘multi-trunk’ trees. As an exception, different genotypes were found for the two trunks of a wild olive tree.
One hundred and ninety-one alleles were amplified with the 14 SSR with 13·64 being the average number of alleles per marker. The mean observed heterozygosity (Ho = 0·740) was higher (range 0·252–0·872) than the expected heterozygosity (He = 0·698), for which values ranged from 0·167 to 0·964. The PIC average value was 0·723 and the accumulated IP of the set of SSR markers was 1·96 × 10−16, ranging between 2·14 × 10−2 for the most informative marker (UDO43) and 5·04 × 10−1 for the least informative marker (DCA15; Supplementary Data Table S1).
Identification of ancient olive genotypes
From the 104 different genotypes discriminated among the a priori cultivated olives, only ten genotypes (9·6 %) matched ten current olive cultivars, and the other 94 genotypes (90·4 %), 49 from the canopy and 45 from the suckers around the base of the trunk, did not match any cultivar. In general, the higher the diameter of the olive (and presumably the older), the higher the proportion of grafted trees and the lower the proportion of trees identified as current cultivars. The cultivars identified among the oldest olives were ‘Gordal Sevillana’, ‘Lechin de Granada’ and ‘Verdial de Velez Malaga’. The largest proportion of ancient trees identified as known cultivars was found in table-olive orchards in the Western provinces. The smallest proportion was observed in south-eastern provinces of Almeria and Granada, where olive trees are grown in dispersed traditional polyculture systems. Strikingly, the presence of grafted trees was higher in these latter provinces and agricultural systems. ‘Lechin de Sevilla’ was the cultivar identified in most trees, followed by ‘Picual’, ‘Gordal Sevillana’ and ‘Verdial de Huevar’.
The identified cultivars were confined to local geographical areas of diffusion, except for ‘Lechin de Sevilla’ and ‘Gordal Sevillana’, which showed a wider diffusion area and thereby were found in different provinces. Notably, 14 previously uncatalogued genotypes were shared by more than one tree, ranging from two to nine trees, depending on the genotype. Nine of these genotypes were found multiple times in different neighbouring orchards in the same province, and one of them was found in orchards located in different provinces.
From the total number of trees (160), 53 (33 %) were not self-rooted. Cultivars, such as ‘Gordal Sevillana’ and ‘Verdial de Velez Malaga’, were always found grafted onto rootstocks, whereas other cultivars, such as ‘Lechin de Granada’ or ‘Lechin de Sevilla’, were found to be either grafted or self-rooted.
Only one supposedly wild ancient olive located in a Roman settlement close to Ubrique, a town in the Sierra of Cadiz (Western area), was identified as a cultivar (‘Lechin de Sevilla’). Also, only one wild olive was grafted, but neither the canopy nor the rootstock genotypes were identified as known olive cultivars.
Genetic relationships among ancient olive genotypes
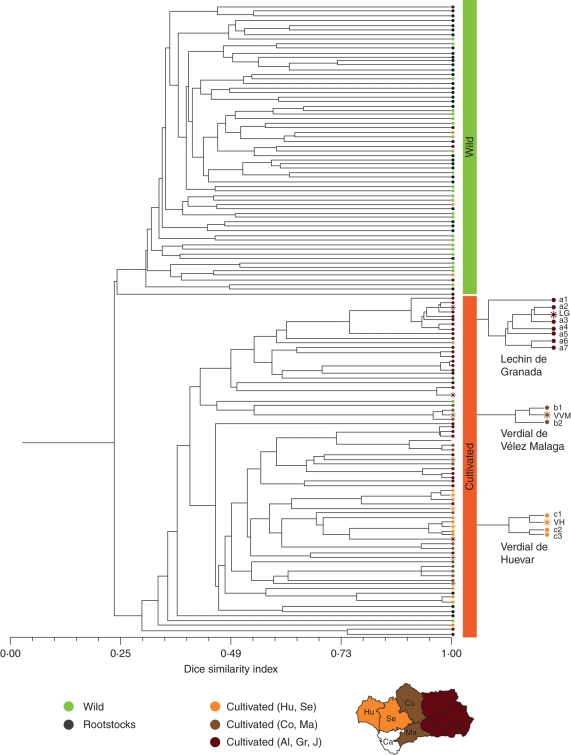
An UPGMA dendrogram based on the Dice similarity index (Dice, 1945) was constructed to study the genetic relationships among the 134 different ancient olive genotypes discriminated by the 14 SSR markers (Fig. 2). This dendrogram was characterized by the early separation of wild and cultivated olives in two different groups sharing a similarity index around 0·22. The first group included mainly wild and rootstock genotypes along with six unidentified olive genotypes that were located in agricultural systems. This set of presumably wild genotypes shared an average similarity index around 0·55 and had no clear geographical association. The second group was composed mainly of genotypes of samples collected from the canopy of cultivated ancient olives. Only three wild and eight rootstock genotypes were included in this group. Of these genotypes, one wild and four rootstock genotypes shared relatively low similarity indexes with the rest of the samples, between 0·29 and 0·36, which were genetically more closely related and showed an average similarity index around 0·75. A subtle geographical pattern of distribution was observed within this second group. Genotypes from the Eastern provinces of Andalusia (Almeria, Granada and Jaen) grouped together as did the genotypes from the Western provinces (Cadiz, Huelva and Seville), and genotypes from the central zone (Cordoba and Malaga) were placed between the two former groups. Finally, twenty-five ancient genotypes shared high similarity indexes (>0·9) with present catalogued olive cultivars. The consistency of these profiles was confirmed by the re-amplification of the samples, which differed only in one or two alleles from their closest cultivar. Three of these cases are described in Fig. 2 and Table 3 for cultivars ‘Lechin de Granada’, ‘Verdial de Velez Malaga’ and ‘Verdial de Huevar’.
Fig. 2.
UPGMA dendrogram based on the Dice similarity index, to study the genetic relationships among the 134 cultivated and wild ancient olive genotypes identified by using 14 SSR markers. Cultivated genotypes are coloured depending on their geographic origin (Al = Almeria, Co = Cordoba, Gr = Granada, H = Huelva, J = Jaen, Ma = Malaga, Se = Sevilla), and those matching current olive cultivars are highlighted with a ‘sun’ symbol. As an example, three possible cases of somatic mutations (genotypes sharing a similarity index >0·9) derived from catalogued cultivars (‘Lechin de Granada’, ‘Verdial de Velez Malaga’ and ‘Verdial de Huevar’) are detailed on the right. The molecular profiles of these samples are shown in Table 3.
Table 3.
SSR profiles of the cultivars ‘Lechin de Granada’, ‘Verdial de Velez Malaga’ and ‘Verdial de Huevar’ and genotypes showing subtle allele differences detected among the cultivated ancient olives
| Genotype | Dca-03 | Dca-09 | Dca-11 | Dca-13 | Dca-15 | Dca-16 | Dca-18 | Gapu-59 | Gapu-71B | Udo-11 | Udo-19 | Udo-24 | Udo-39 | Udo-43 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Lechin de Granada’ | 237/243 | 182/204 | 140/178 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/134 | 129/129 | 164/164 | 176/176 | 212/216 |
| a 1 | 237/243 | 182/204 | 140/178 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/134 | 129/129 | 164/164 | 176/176 | 206/212 |
| a 2 | 237/243 | 182/204 | 140/178 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/134 | 129/129 | 164/164 | 176/176 | 212/218 |
| a 3 | 237/243 | 182/204 | 140/174 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/134 | 129/129 | 164/164 | 176/176 | 212/216 |
| a 4 | 237/243 | 182/194 | 140/178 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/134 | 129/129 | 164/164 | 176/176 | 212/216 |
| a 5 | 237/243 | 182/204 | 140/178 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/131 | 129/129 | 164/164 | 176/176 | 212/216 |
| a 6 | 237/243 | 174/204 | 140/178 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/134 | 129/129 | 164/164 | 176/176 | 212/214 |
| a 7 | 237/243 | 182/204 | 140/178 | 116/116 | 254/254 | 122/152 | 166/168 | 210/220 | 121/127 | 116/134 | 129/129 | 164/164 | 176/176 | 208/216 |
| ‘Verdial de Velez Malaga’ | 241/243 | 174/192 | 146/178 | 116/116 | 243/243 | 152/175 | 168/172 | 210/220 | 124/141 | 116/134 | 97/129 | 164/164 | 163/181 | 172/216 |
| b 1 | 241/243 | 174/192 | 146/178 | 116/116 | 243/243 | 152/175 | 168/172 | 210/220 | 124/141 | 116/129 | 97/129 | 164/164 | 163/181 | 172/216 |
| b 2 | 241/243 | 174/192 | 146/178 | 116/116 | 243/243 | 152/175 | 168/172 | 210/220 | 121/141 | 116/134 | 97/129 | 164/164 | 163/181 | 172/216 |
| ‘Verdial de Huevar’ | 237/247 | 182/192 | 140/160 | 118/136 | 264/264 | 152/175 | 168/176 | 210/220 | 118/121 | 116/119 | 129/129 | 164/185 | 181/181 | 172/212 |
| c 1 | 237/247 | 182/192 | 140/160 | 118/136 | 264/264 | 152/179 | 168/176 | 210/220 | 118/121 | 116/119 | 129/129 | 164/185 | 181/181 | 172/212 |
| c 2 | 237/247 | 182/192 | 140/160 | 118/136 | 264/264 | 152/177 | 168/176 | 210/220 | 118/121 | 116/119 | 129/129 | 164/185 | 181/181 | 172/212 |
| c 3 | 237/247 | 182/192 | 140/160 | 118/136 | 243/264 | 152/175 | 168/176 | 210/220 | 118/121 | 116/119 | 129/129 | 164/185 | 181/181 | 172/214 |
Differences in alleles between the genotypes and their closest cultivar are highlighted in bold.
Population structure of ancient olives
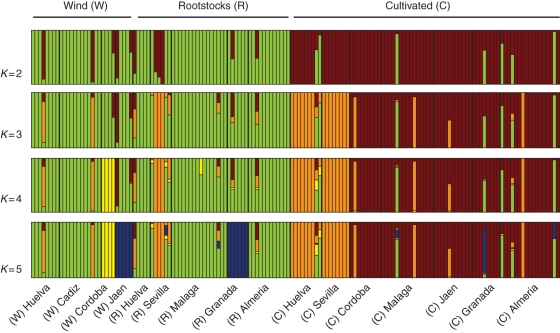
The Bayesian approach implemented in BAPS was applied to search for hidden population structure among ancient genotypes (Fig. 3). The most likely number of genetic clusters inferred by BAPS was K = 5 (highest posterior probability). Structure software was applied to check BAPS results, and a bimodal shape was observed for the ΔK distribution, indicating the most likely number of genetic clusters at K = 2 and K = 5 (data not shown).
Fig. 3.
Inference of population structure among ancient olive genotypes grouped according to geographical origin, assuming K = 2 to K = 5 subpopulations. Each strain is represented by a single vertical bar that is partitioned into K coloured segments that represent the strain's estimated ancestry proportion in each of the K clusters.
Both Bayesian approaches identified similar clusters at the individual level. As shown in Fig. 3, at K = 2 a clear separation between cultivated and wild-olive genotypes (red and green colours, respectively) was observed, with rootstock genotypes being included in the same genetic group as wild olives. Only 19 genotypes appeared as mosaics that are hybrids between cultivated and wild clusters. At K = 3, the wild genetic cluster remained unchanged, but the cultivated cluster split out into two groups, one clustering the majority of the olives from the Western provinces (Huelva and Seville, coloured orange) and the other gathering the rest of the cultivars from the Central and Eastern provinces. The genotypes of rootstocks from Seville also belong totally or partially to this third cluster. At K = 4, wild olives from one of the Central provinces (Cordoba, coloured yellow) appeared as a new genetic cluster. Five genotypes from Seville, three rootstocks and two cultivated), had a low admixture coefficient in this cluster. At K = 5, the cultivated clusters remained unchanged, but a new cluster gathering the wild and rootstock genotypes from Eastern provinces (Jaen and Granada, coloured blue) appeared. Three cultivated samples from Almeria, Granada (Eastern) and Malaga (Central) presented a coefficient of admixture in this last cluster.
DISCUSSION
Overall genetic diversity
The set of SSR markers employed in this study has allowed the genetic characterization of the ancient olives of Andalusia. This set of SSRs is systematically used in the WOGB of Cordoba to identify new olive accessions (I. Trujillo, University of Cordoba, Spain, unpubl. res.) and has been also successfully employed in previous studies carried out in olives (Noormohammadi et al., 2007; Bracci et al., 2009; Belaj et al., 2010; Erre et al., 2010).
Polymorphism levels were similar to those obtained in previous studies aimed at evaluating the genetic diversity and relationships among wild and cultivated olives by different kinds of molecular markers such as isozymes (Lumaret et al., 2004), RAPDs (Bronzini de Caraffa et al., 2002a, b), AFLPs (Angiolillo et al., 1999; Baldoni et al., 2006); and SSRs (Breton et al., 2006; Belaj et al., 2007, 2010; Erre et al., 2010). Mean heterozygosity values (Ho = 0·740 and He = 0·698) were similar to those reported by other authors studying sets of cultivated and wild olives by means of SSR (Breton et al., 2006; Belaj et al., 2010; Erre et al., 2010). The total cumulative PI value for this set of markers was 1·96 × 10−16, which shows the high discrimination power of the selected set of primers.
What is the identity of ancient olives?
Only a small number of cultivated ancient olive genotypes were identified as known cultivars, specifically ten (9·6 %). out of the 104 different genotypes characterized among the a priori cultivated olives. This result supports those obtained in previous studies in which ancient olive trees from Italy were genotyped and only a reduced proportion of them matched current olive cultivars (Baldoni et al., 2006; Erre et al., 2010). Consistent with previous studies that indicate traditional olives are confined in their putative domestication areas (Claros et al., 2000; Besnard et al., 2001; Barazani et al., 2008), eight out of ten identified cultivars were sampled only in one province previously described as their putative area of origin (Barranco and Rallo, 1984; Barranco et al., 2005). Only two identified cultivars, ‘Lechin de Sevilla’ and ‘Gordal Sevillana’, were found in wider olive growing areas, which may be due to their valuable agronomic characteristics. ‘Lechin de Sevilla’ has an elevated adaptation to unfavorable lands and resistance to olive leaf spot caused by Spilocaea oleagina (Trapero and Lopez Doncel, 2005), and ‘Gordal Sevillana’ is widely known for its exceptional size and value as a table olive (Barranco et al., 2005).
The major proportion (90·4 %) of the a priori cultivated ancient olive genotypes did not match any current cultivar, despite the fact that the Spanish catalogue of olive cultivars is one of the most detailed and exhaustive in any traditional olive growing country (Barranco and Rallo, 2000; Barranco et al., 2005). These unknown ancient genotypes were also linked to restricted geographical areas and could be traditional cultivars that evaded previous surveys and have remained uncatalogued throughout time. Consequently, they represent an unexploited reservoir of olive genetic diversity. The domesticated status of the greater part of these uncatalogued ancient genotypes may be inferred by three main observations: (1) the majority of these genotypes grouped together with the current olive cultivars in this study (Fig. 2); (2) 25 genotypes were grafted onto rootstocks; and (3) 14 genotypes were represented by more than one tree. These uncatalogued ancient cultivars should be propagated and conserved ex situ before their morphological description and agronomic evaluation. Notably, many of these trees were located in traditional groves in the Eastern provinces that have extremely dry climates with an average rainfall of around 250 mm year−1 (Red de Informacion Agroclimatica de Andalucia, 2010). The outstanding performance of these trees makes their agronomical evaluation and possible use in olive breeding programmes especially useful.
As expected, higher similarity indexes were observed among cultivated genotypes compared with wild genotypes (Lumaret et al., 2004; Belaj et al., 2010). The domestication process entails a selective bottleneck and a shift towards fixing alleles related to valuable agronomic traits (for a review, see Doebley et al., 2006). As currently occurs in modern breeding programmes, new cultivars were more likely to be obtained by traditional farmers from seedlings in cultivated groves and, therefore, were originated by crossing cultivated forms rather than from local selection of wild genotypes.
The population structure analysis showed two clear genetic clusters among the cultivated ancient olives at K = 3 (Fig. 3) that were also clustered by the UPGMA dendrogram (Fig. 2). This structure could be due to the different uses of the main olive cultivars in each area, as was reported in previous studies (Claros et al., 2000; Besnard et al., 2001; Hagidimitriou et al., 2005). The Western provinces (Huelva and Sevilla, Cluster 3) are the main table-olive producer and the rest of Andalusia has traditionally focused on oil production. Actually, the majority of the ancient olives sampled in the Western provinces are still harvested for table-olive consumption.
On the antiquity of the cultivars
Traditional olive cultivars were likely to have been selected by local farmers for extensive cultivation under dry conditions (Zohary and Spiegel Roy, 1975; Rallo, 2005), but nothing is known about their antiquity. The largest in size and, presumably, the most ancient olives matched three current cultivars: ‘Gordal Sevillana’, ‘Lechin de Granada’ and ‘Verdial de Velez Malaga’. The former cultivar is one of the most widely known table olive cultivars and is typically found in the Western region of Andalusia, specifically the Sevilla province, an area that has been devoted to oil and table olive production since the Roman period (I and II centuries ad) (Remesal-Rodriguez, 2008). The other two cultivars, ‘Lechin de Granada’ and ‘Verdial de Velez Malaga’, were found in polyculture systems that were mainly developed during the Arab period (VIII–XIV centuries ad) in mountainous regions of the Eastern (Almeria and Granada) and Central (Malaga) areas of Andalusia (Trillo-San Jose, 2008). Additionally, archaeological evidence supports the antiquity of wild-olive use in this area since Neolithic times (Terral and Mengual, 1999; Rodriguez-Ariza and Moya, 2005), where traditional agricultural systems that are still present have allowed the conservation of old olives and ancient cultivars (Claros et al., 2000; Muñoz-Diez et al., 2004). Strikingly, the cultivar ‘Picual’, at present the main cultivar of Andalusia, was only identified among the smallest trees and only in the province of Jaen, its original area of distribution (Barranco and Rallo, 2000). Nevertheless, the relatively recent historic expansion of this cultivar supports this fact (Guzmán-Álvarez, 2004). In general, the percentage of ancient olives that did not match current cultivars was highest among the oldest olives. This result indicates that the oldest long-lived trees are reservoirs of genetic diversity preserved by traditional agricultural systems that still remain in the south of Spain.
The percentage of grafted trees was higher among the trees with the largest trunk diameter, which suggests that this technique was used more frequently in the past to convert pre-existing wild olives into cultivated ones. Wild rootstocks can confer hardiness to the grafted cultivar and ensure the adaptation of the root system to the environment (Barranco, 2008). Wild olive trees with the largest trunk diameter were situated in the Western provinces (Cadiz and Huelva), where large and undisturbed wild-olive forests existed as part of the indigenous Mediterranean vegetation (Rubio de Casas et al., 2006; Belaj et al., 2010).
Somatic mutations and ancient olives
Subtle genetic differences (similarity index >0·9) were observed among several genotypes (Fig. 2 and Table 3). These small differences could be due to somatic point mutations rather than to outcrossing among cultivars as they slightly differ from their closest cultivars. However, the possibility of genotyping errors has to be taken into account. Somatic mutations have previously been reported in olives by using different molecular markers such as RAPD, AFLP and SSR (Cipriani et al., 2002; Banilas et al., 2003; García-Díaz et al., 2003; Charafi et al., 2008; Mazzalupo et al., 2010). Sampling surveys aimed to look for phenotypic diversity within olive cultivars showed that intracultivar phenotypic diversity was an unusual phenomenon in olive (Belaj et al., 2004).
The degree of correlation between somatic point mutations and phenotypic variation remains in question, especially because a different genotype is not considered to be a new cultivar until its unique phenotypic and agronomic performance has been verified (International Union for the Protection of New Varieties of Plants, 1991). Mosaicism (intraorganismal genetic heterogeneity) is most likely to occur in highly variable and neutrally evolving genomic regions like SSRs, which can accumulate mutations without necessary phenotypic consequences in crop morphology and agronomic performance (Gill et al., 1995; Regner et al., 2000a, b; Franks et al., 2002; Crespan, 2004). Additionally, mosaicism could be more frequent during the senescent phase of a tree because the mutation rate increases during this period (Petit and Hampe, 2006). Notably, these subtle genetic differences among genotypes were mostly observed in cultivars like ‘Lechin de Granada’ and ‘Verdial de Velez Malaga’, which were identified among the biggest and, presumably, oldest olives. Further studies are required to disentangle the various factors affecting the evolution of clonally propagated fruit crops (Pineda-Krch and Lehtilä, 2004; Mckey et al., 2010). Future analysis of ancient olive trees could play a key role in answering this question and how and at which frequency new cultivars can arise by somatic point mutations.
Were the first olive orchards developed from olive forests?
Grafting, described as ‘instant domestication’ and developed >3800 years ago (Harris et al., 2002), was surely used by farmers to transform autochthonous wild olive forests into primitive olive groves characterized by uneven distances between the trees. The putative wild status of the majority of the rootstocks detected in this study (53 out of 160 trees were grafted) was supported by their clustering together with the wild ancient olives in the UPGMA dendrogram (Fig. 2). Additionally, two clear groups were observed at K = 2 in the genetic structure analysis (Fig. 3), one including the cultivated genotypes and the other the majority of the wild and rootstock genotypes. The present results are in agreement with those obtained by Barazani et al. (2008) in Israel and confirm on a larger scale those obtained by Baldoni et al. (2006) and Erre et al. (2010), suggesting that the beginnings of olive growing in some areas of the West Mediterranean Basin were also based on the development of grafting techniques. Grafting has also been used for horticultural purposes such as tolerance to root anoxia by mean of the use of a tolerant cultivar (‘Verdial de Huevar’) and for growing bad rooting cultivars as ‘Gordal Sevillana’ and ‘Verdial de Velez Málaga’ (Barranco et al., 2005; Barranco, 2008). The propagation techniques in the past using hard-cuttings may explain the grafting of the same genotype onto the same rootstock in ‘multi-trunk’ grafted trees. Probably, several sprouts coming from the same hard-cutting were selected and grafted with the desired cultivar. Another plausible hypothesis is that pre-existing wild olives composed by several trunks, coming up from the same root system, were used to graft the desired cultivar. Contrastingly, the fact that one wild olive tree was composed of two genetically differentiated trunks could be due to the germination of two different seeds close to each other appearing as a single tree.
Adaptation to local environmental conditions could also explain the subdivision of wild olives into three different genetic clusters according to the structure analysis at K = 5. Actually, the coastal regions of Western Andalusia (Huelva and Seville) are warmer and more humid than most of the Eastern provinces (Jaen and Granada), and the climatologic conditions of the Central region (Cordoba) show intermediate characteristics. Similar impacts of these climatic factors on the population structure of wild olives have been observed previously (Belaj et al., 2007). Although the analysis of a larger number of wild-olive samples from different geographical areas would clarify better the possible contribution of the climatologic conditions on the genetic diversity patterns of wild olive populations.
Conclusions
This study of ancient olives has been fruitful both for germplasm collection and for increasing our knowledge about olive domestication. The findings suggest that grafting pre-existing wild olives with olive cultivars was linked to the beginnings of olive growing. Additionally, the low number of genotypes identified with current cultivars indicated that ancient olives from southern Spain constitute a priceless reservoir of genetic diversity. Further studies including an extended number of current cultivars and wild olives of the same region might be able to assess the ‘paleocultivar’ status of these ancient genotypes. On the basis of these results the ancient olives of the South of Spain deserve to be considered an in-situ collection of olive genetic resources.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was mainly supported by the Consejeria de Economia, Innovacion y Ciencia of the regional government of Andalusia and the Instituto Nacional de Investigacion Agrarias (INIA) via funds from Project P09-AGR-5010 and Project RF2006–00017-C02-01, respectively. We thank B. S. Gaut and his group at the Ecology and Evolutionary Biology Department of the University of California–Irvine for their helpful comments and suggestions on the manuscript. A. Belaj acknowledges a post-doctoral INIA contract (Subprograma DOC-INIA). The authors specially acknowledge all the persons and institutions that collaborate in the location and conservation of the centennial olive trees of Andalusia, Spain.
LITERATURE CITED
- Angiolillo A, Mencuccini M, Baldoni L. Olive genetic diversity assessed using amplified fragment length polymorphisms. Theoretical and Applied Genetics. 1999;98:411–421. [Google Scholar]
- Baldoni L, Cultrera NG, Mariotti R, et al. A consensus list of microsatellite markers for olive genotyping. Molecular Breeding. 2009;24:213–231. [Google Scholar]
- Baldoni L, Tosti N, Ricciolini C, et al. Genetic structure of wild and cultivated olives in the central Mediterranean basin. Annals of Botany. 2006;98:935–942. doi: 10.1093/aob/mcl178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banilas G, Minas J, Gregoriou C, Demoliou C, Kourti A, Hatzopoulos P. Genetic diversity among accessions of an ancient olive variety of Cyprus. Genome. 2003;46:370–376. doi: 10.1139/g03-011. [DOI] [PubMed] [Google Scholar]
- Barazani A, Dag A, Kerem Z, Lavee S, Kadereit J. Local old olive landrace varieties in Israel: valuable plant genetic resources in olive cultivation. Israel Journal of Plant Sciences. 2008;56:265–271. [Google Scholar]
- Barranco D. Variedades y Patrones. In: Barranco D, Fernandez-Escobar R, Rallo L, editors. El cultivo del olivo. 6th edn. Madrid: Mundi-Prensa y Junta de Andalucia; 2008. pp. 37–62. [Google Scholar]
- Barranco D, Rallo L. Las variedades de olivo cultivadas en Andalucia. Madrid, Spain: Ministerio de Agricultura, Junta de Andalucia; 1984. [Google Scholar]
- Barranco D, Rallo L. Olive cultivars in Spain. HortTechnology. 2000;10:107–110. [Google Scholar]
- Barranco D, Trujillo I, Rallo L. Elaiografía Hispanica. In: Rallo L, Caballero JM, Del Rio C, Martin A, Tous J, Trujillo I, editors. Variedades de olivo en España. Madrid: Junta de Andalucía, MAPA y Ediciones Mundi-Prensa; 2005. pp. 45–231. [Google Scholar]
- Belaj A, Rallo L, Trujillo I, Baldoni L. Using RAPD and AFLP markers to distinguish individuals obtained by clonal selection of ‘Arbequina’ and ‘Manzanilla de Sevilla’ olive. Hortscience. 2004;39:1566–1570. [Google Scholar]
- Belaj A, Muñoz-Díez C, Baldoni L, Porceddu A, Barranco D, Satovic Z. Genetic diversity and population structure of wild olives from the north-western Mediterranean assessed by SSR markers. Annals of Botany. 2007;100:449–458. doi: 10.1093/aob/mcm132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaj A, Muñoz-Díez C, Baldoni L, Satovic Z, Barranco D. Genetic diversity and relationships of wild and cultivated olives at regional level in Spain. Scientia Horticulturae. 2010;124:323–330. [Google Scholar]
- Besnard G, Baradat P, Berville A. Genetic relationships in the olive (Olea europaea L.) reflect multilocal selection of cultivars. Theoretical and Applied Genetics. 2001;102:251–258. [Google Scholar]
- Botstein D, White RL, Skolnick M, Davis RW. Construction of a genetic-linkage map in man using restriction fragment length polymorphisms. American Journal of Human Genetics. 1980;32:314–331. [PMC free article] [PubMed] [Google Scholar]
- Bracci T, Sebastiani L, Busconi M, Fogher C, Belaj A, Trujillo I. SSR markers reveal the uniqueness of olive cultivars from the Italian region of Liguria. Scientia Horticulturae. 2009;122:209–215. [Google Scholar]
- Breton C, Tersac M, Berville A. Genetic diversity and gene flow between the wild olive (oleaster, Olea europaea L.) and the olive: several Plio-Pleistocene refuge zones in the Mediterranean basin suggested by simple sequence repeats analysis. Journal of Biogeography. 2006;33:1916–1928. [Google Scholar]
- Bronzini de Caraffa V, Giannettini J, Gambotti C, Maury J. Genetic relationships between cultivated and wild olives of Corsica and Sardinia using RAPD markers. Euphytica. 2002a;123:263–271. [Google Scholar]
- Bronzini de Caraffa V, Maury J, Gambotti C, Breton C, Berville A, Giannettini J. Mitochondrial DNA variation and RAPD mark oleasters, olive and feral olive from Western and Eastern Mediterranean. Theoretical and Applied Genetics. 2002b;104:1209–1216. doi: 10.1007/s00122-002-0883-7. [DOI] [PubMed] [Google Scholar]
- Burley AL, Phillips S, Ooi MKJ. Can age be predicted from diameter for the obligate seeder Allocasuarina littoralis (Casuarinaceae) by using dendrochronological techniques? Australian Journal of Botany. 2007;55:433–438. [Google Scholar]
- Charafi J, El Meziane A, Moukhli A, Boulouha B, El Modafar C, Khadari B. Menara gardens: a Moroccan olive germplasm collection identified by a SSR locus-based genetic study. Genetic Resources and Crop Evolution. 2008;55:893–900. [Google Scholar]
- Cipriani G, Marrazzo MT, Marconi R, Cimato A, Testolin R. Microsatellite markers isolated in olive (Olea europaea L.) are suitable for individual fingerprinting and reveal polymorphism within ancient cultivars. Theoretical and Applied Genetics. 2002;104:223–228. doi: 10.1007/s001220100685. [DOI] [PubMed] [Google Scholar]
- Claros MG, Crespillo R, Aguilar ML, Canovas FM. DNA fingerprinting and classification of geographically related genotypes of olive-tree (Olea europaea L.) Euphytica. 2000;116:131–142. [Google Scholar]
- Corander J, Marttinen P. Bayesian identification of admixture events using multilocus molecular markers. Molecular Ecology. 2006;15:2833–2843. doi: 10.1111/j.1365-294X.2006.02994.x. [DOI] [PubMed] [Google Scholar]
- Corander J, Waldmann P, Sillanpaa MJ. Bayesian analysis of genetic differentiation between populations. Genetics. 2003;163:367–374. doi: 10.1093/genetics/163.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corander J, Marttinen P, Siren J, Tang J. Enhanced Bayesian modelling in BAPS software for learning genetic structures of populations. BMC Bioinformatics. 2008;9:539. doi: 10.1186/1471-2105-9-539. doi:10.1186/1471-2105-9-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespan M. Evidence on the evolution of polymorphism of microsatellite markers in varieties of Vitis vinifera L. Theoretical and Applied Genetics. 2004;108:231–237. doi: 10.1007/s00122-003-1419-5. [DOI] [PubMed] [Google Scholar]
- Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- Doebley JF, Gaut BS, Smith BD. The molecular genetics of crop domestication. Cell. 2006;127:1309–1321. doi: 10.1016/j.cell.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Elbaum R, Melamed-Bessudo C, Boaretto E, Galili E, Lev-Yadun S, Levy AA, Weiner S. Ancient olive DNA in pits: preservation, amplification and sequence analysis. Journal of Archaeological Science. 2006;33:77–88. [Google Scholar]
- Erre P, Chessa I, Muñoz-Diez C, Belaj A, Rallo L, Trujillo I. Genetic diversity and relationships between wild and cultivated olives (Olea europaea L.) in Sardinia as assessed by SSR markers. Genetic Resources and Crop Evolution. 2010;57:41–54. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Franks T, Botta R, Thomas MR. Chimerism in grapevines: implications for cultivar identity, ancestry and genetic improvement. Theoretical and Applied Genetics. 2002;104:192–199. doi: 10.1007/s001220100683. [DOI] [PubMed] [Google Scholar]
- Garcia-Diaz A, Oya R, Sanchez A, Luque F. Effect of prolonged vegetative reproduction of olive tree cultivars (Olea europaea L.) in mitochondrial homoplasmy and heteroplasmy. Genome. 2003;46:377–381. doi: 10.1139/g03-017. [DOI] [PubMed] [Google Scholar]
- Gill DE, Chao L, Perkins SL, Wolf JB. Genetic mosaicism in plants and clonal animals. Annual Review of Ecology and Systematics. 1995;26:423–444. [Google Scholar]
- Guzman-Alvarez JR. El palimpsesto cultivado: historia de los paisajes del olivar andaluz. Sevilla: Consejeria de Agricultura y Pesca; 2004. [Google Scholar]
- Hagidimitriou M, Katsiotis A, Menexes G, Pontikis C, Loukas M. Genetic diversity of major Greek olive cultivars using molecular (AFLPs and RAPDs) markers and morphological traits. Journal of the American Society for Horticultural Science. 2005;130:211–217. [Google Scholar]
- Hansson MC, Foley BP. Ancient DNA fragments inside Classical Greek amphoras reveal cargo of 2400-year-old shipwreck. Journal of Archaeological Science. 2008;35:1169–1176. [Google Scholar]
- Harris SA, Robinson JP, Juniper BE. Genetic clues to the origin of the apple. Trends in Genetics. 2002;18:426–430. doi: 10.1016/s0168-9525(02)02689-6. [DOI] [PubMed] [Google Scholar]
- International Olive Council. 2010 Statistical series. http://www.internationaloliveoil.org/estaticos/view/131-world-olive-oil-figures. (accessed 6 February 2011) [Google Scholar]
- International Union for the Protection of New Varieties of Plants. 1991 International convention for the protection of new varieties of plants of 2 December 1961, as revised at Geneva on 10 November 1972, on 23 October 1978 and on 19 March 1991. http://www.upov.int/en/publications. (accessed 6 February 2011) [Google Scholar]
- Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–1806. doi: 10.1093/bioinformatics/btm233. [DOI] [PubMed] [Google Scholar]
- Liu KJ, Muse SV. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- Lumaret R, Ouazzani N, Michaud H, Vivier G, Deguilloux MF, Di Giusto F. Allozyme variation of oleaster populations (wild olive tree) (Olea europaea L.) in the Mediterranean Basin. Heredity. 2004;92:343–351. doi: 10.1038/sj.hdy.6800430. [DOI] [PubMed] [Google Scholar]
- McKey D, Elias M, Pujol B, Duputie A. The evolutionary ecology of clonally propagated domesticated plants. New Phytologist. 2010;186:318–332. doi: 10.1111/j.1469-8137.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- Mazzalupo I, Chiappetta A, Benicasa C, Perri E. Intra-cultivar variability of three major olive cultivars grown in different areas of central-southern Italy and studies using microsatellite markers. Scientia Horticulturae. 2010;126:324–329. [Google Scholar]
- Montemurro C, Simeone R, Pasqualone A, Ferrara E, Blanco A. Genetic relationships and cultivar identification among 112 olive accessions using AFLP and SSR markers. Journal of Horticultural Science & Biotechnology. 2005;80:105–110. [Google Scholar]
- Muñoz-Díez C. Spain; 2008. Prospección, diversidad genética y conservación de ejemplares monumentales y poblaciones silvestres de olivo (Olea europaea L.). PhD Thesis, University of Cordoba. [Google Scholar]
- Muñoz-Díez C, Belaj A, Barranco D, Rallo L. Olivos monumentales de España. Madrid: Mundiprensa-UnoEdiciones; 2004. [Google Scholar]
- Nikoloudakis N, Banilas G, Gazis F, Hatzopoulos P, Metzidakis J. Discrimination and genetic diversity among cultivated olives of Greece using RAPD markers. Journal of the American Society for Horticultural Science. 2003;128:741–746. [Google Scholar]
- Noormohammadi Z, Hosseini-Mazinani M, Trujillo I, Ratio L, Belaj A, Sadeghizadeh M. Identification and classification of main Iranian olive cultivars using microsatellite markers. Hortscience. 2007;42:1545–1550. [Google Scholar]
- van Oosterhout C, Weetman D, Hutchinson WF. Estimation and adjustment of microsatellite null alleles in nonequilibrium populations. Molecular Ecology Notes. 2006;6:255–256. [Google Scholar]
- Paetkau D, Strobeck C. Microsatellite analysis of genetic-variation in black bear populations. Molecular Ecology. 1994;3:489–495. doi: 10.1111/j.1365-294x.1994.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Hampe A. Some evolutionary consequences of being a tree. Annual Review of Ecology, Evolution and Systematics. 2006;37:187–214. [Google Scholar]
- Pineda-Krch M, Lehtilä K. Costs and benefits of genetic heterogeneity within organisms. Journal of Evolutionary Biology. 2004;17:1167–1177. doi: 10.1111/j.1420-9101.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rallo L. Variedades de olivo en España: una aproximación cronológica. In: Rallo L, Caballero JM, Del Rio C, Martin A, Tous J, Trujillo I, editors. Variedades de olivo en España. Madrid: Junta de Andalucía, MAPA y Ediciones Mundi-Prensa; 2005. pp. 17–44. [Google Scholar]
- Rallo L, Muñoz-Díez C. Olive growing in a time of change. In: Verheye H, editor. Soils, plant growth and crop production. 2010. In: Encyclopedia of life support systems (EOLSS), developed under the auspices of the UNESCO. Oxford: Eolss Publishers. http://www.eolss.net. (accessed 8 February 2011) [Google Scholar]
- Red de Informacion Agroclimatica de Andalucia. 2010 Series de datos climaticos. Sevilla: Consejeria de Agricultura y Pesca de Andalucia web. http://www.juntadeandalucia.es/agriculturaypesca/ifapa/ria/servlet/FrontController. (accessed 6 February 2011) [Google Scholar]
- Regner F, Stadlbauer A, Eisenheld C, Kaserer H. Genetic relationships among Pinots and related cultivars. American Journal of Enology and Viticulture. 2000a;51:7–14. [Google Scholar]
- Regner F, Wiedeck E, Stadlbauer A. Differentiation and identification of White Riesling clones by genetic markers. Vitis. 2000b;39:103–107. [Google Scholar]
- Remesal-Rodriguez J. Tierras del olivo. Granada: El Legado Andalusi; 2008. El aceite bético en el imperio romano; pp. 66–81. [Google Scholar]
- Rodriguez-Ariza MO, Moya EM. On the origin and domestication of Olea europaea L. (olive) in Andalusia, Spain, based on the biogeographical distribution of its finds. Vegetation History and Archaeobotany. 2005;14:551–561. [Google Scholar]
- Rohlf FJ. NTSYS-pc. Numerical taxonomy and multivariate analysis system, version 2·00. New York, NY: Exeter Software, Setauket; 1998. [Google Scholar]
- de la Rosa R, James C, Tobutt KR. Isolation and characterization of polymorphic microsatellites in olive Olea europaea L. and their transferability to other genera in the Oleaceae. Molecular Ecology Notes. 2002;2:265–267. [Google Scholar]
- Rosenberg NA. DISTRUCT: a program for the graphical display of population structure. Molecular Ecology Notes. 2004;4:137–138. [Google Scholar]
- Rozas V. Tree age estimates in Fagus sylvatica and Quercus robur: testing previous and improved methods. Plant Ecology. 2003;167:193–212. [Google Scholar]
- Rozas V. A dendroecological reconstruction of age structure and past management in an old-growth pollarded parkland in northern Spain. Forest Ecology and Management. 2004;195:205–219. [Google Scholar]
- Rubio de Casas R, Besnard G, Schoenswetter P, Balaguer L, Vargas P. Extensive gene flow blurs phylogeographic but not phylogenetic signal in Olea europaea L. Theoretical and Applied Genetics. 2006;113:575–583. doi: 10.1007/s00122-006-0306-2. [DOI] [PubMed] [Google Scholar]
- Terral JF. Exploitation and management of the olive tree during prehistoric times in Mediterranean France and Spain. Journal of Archaeological Science. 2000;27:127–133. [Google Scholar]
- Terral JF, Mengual X. Reconstruction of Holocene climate in southern France and eastern Spain using quantitative anatomy of olive wood and archaeological charcoal. Palaeogeography, Palaeoclimatology, Palaeoecology. 1999;153:71–92. [Google Scholar]
- Trapero A, Lopez Doncel LM. Resistencia y susceptibilidad al repilo. In: Rallo L, Caballero JM, Del Rio C, Martin A, Tous J, Trujillo I, editors. Variedades de olivo en España. Madrid: Junta de Andalucía, MAPA y Ediciones Mundi-Prensa; 2005. pp. 321–328. [Google Scholar]
- Trillo-San Jose C. Tierras del olivo. Granada: El Legado Andalusi; 2008. El olivo en Al-Andalus: tradición latina e islamica; pp. 104–115. [Google Scholar]
- Valiere N. GIMLET: a computer program for analysing genetic individual identification data. Molecular Ecology Notes. 2002;2:377–379. [Google Scholar]
- Zohary D, Spiegel-Roy P. Beginnings of fruit growing in the Old World. Science. 1975;187:319–327. doi: 10.1126/science.187.4174.319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.