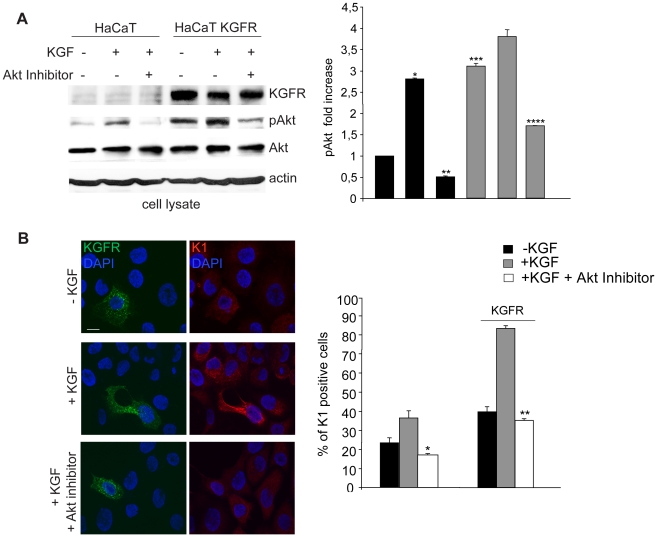
Figure 7. The PI3K/Akt signaling pathway is involved in KGFR-mediated early differentiation.
(A) HaCaT KGFR and HaCaT cells were serum starved for 4 h and treated with 100 ng/ml KGF for 10′ at 37°C. Western blot analysis using anti-Bek and anti-phospho-Akt polyclonal antibodies shows that Akt protein phosphorylation is evident following KGF treatment and it is specifically blocked by the Akt inhibitor in HaCaT control cells. In KGFR transfected cells, the Akt phosphorylation is more intense and clearly detectable also in serum-free untreated cells. The specific band corresponding to the receptor is well visible only in KGFR transfected cells. The equal loading was assessed with anti-actin antibody. For the densitometric analysis of band corresponding to phospho-Akt protein band the values from three independent experiments were normalized, expressed as fold increase and reported in graph as mean values +/− SE. Student's t test was performed and significance level has been defined as *p<0.001 vs the corresponding KGF-untreated cells, **p<0.005 vs the corresponding Akt inhibitor-untreated cells, ***p<0.001 vs the corresponding untransfected cells and ****p<0.005 vs the corresponding Akt inhibitor-untreated cells. (B) HaCaT KGFR and HaCaT cells were serum starved for 12 h and then stimulated with 20 ng/ml KGF for the last 24 h at 37°C in presence or not of the specific Akt inhibitor. Quantitative immunofluorescence analysis shows that the Akt inhibitor drastically blocks the increase of K1 positive cells observed upon KGFR overexpression and KGF stimulation. The Akt inhibitor decreases the KGF-mediated differentiation also in cells expressing endogenous levels of the receptor. Results are expressed as mean values ± SE. Student's t test was performed and significance level has been defined as *p<0.001 and **p<0.001 vs the corresponding, KGF-treated cells. Bar, 10 µm.