Abstract
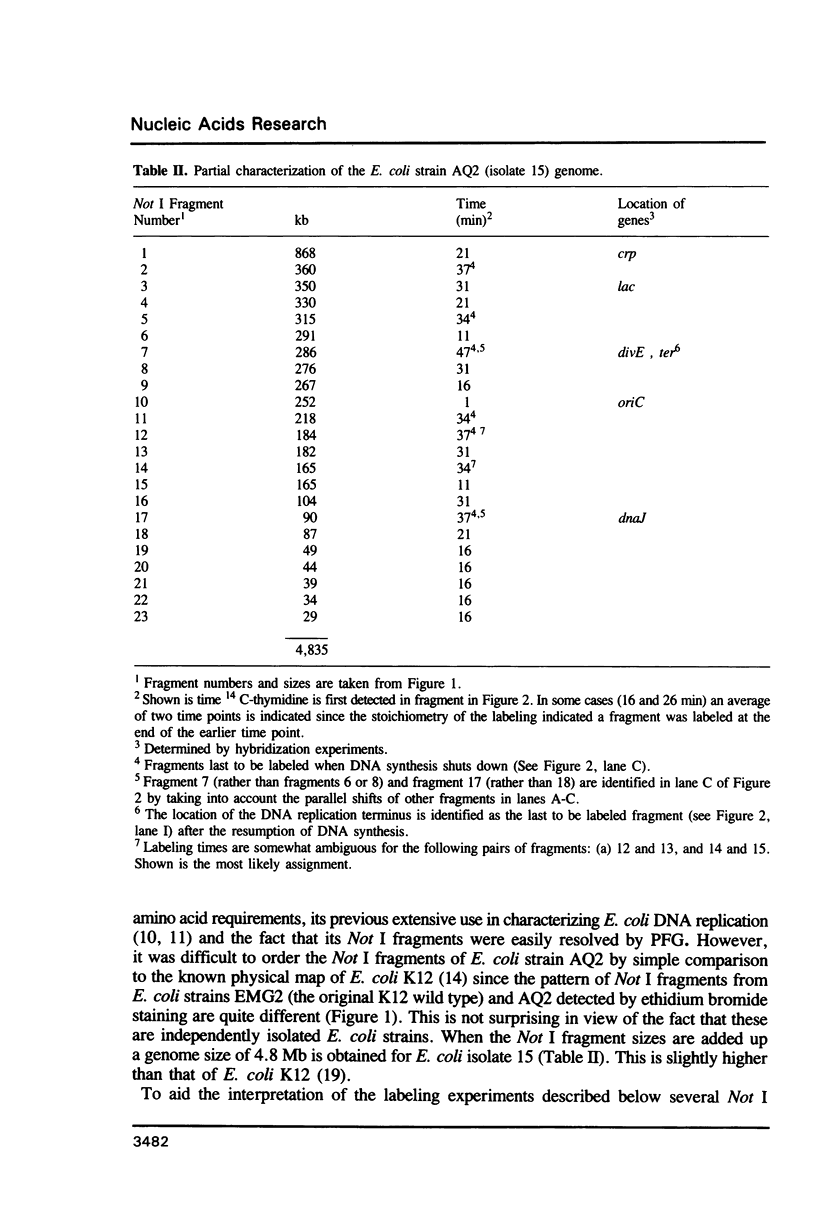
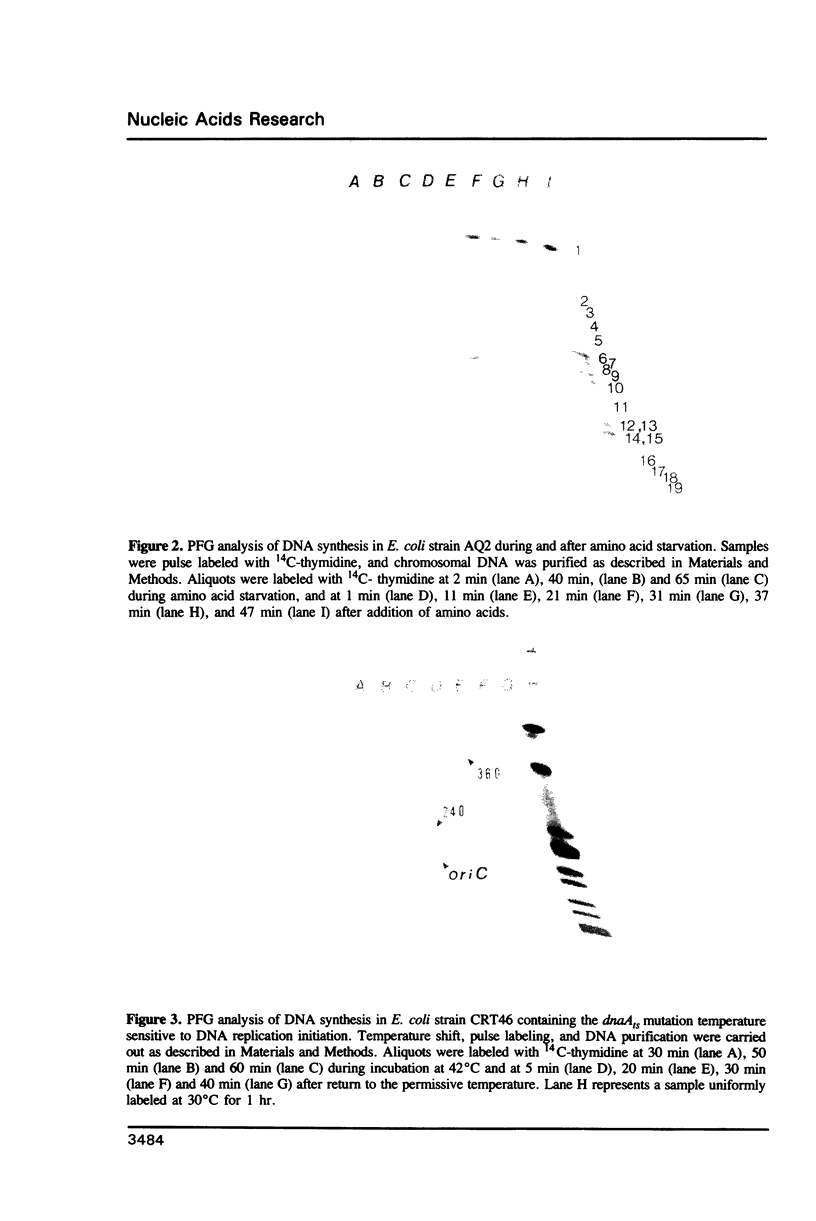
The location of chromosomal DNA replication forks was identified in synchronously replicating E. coli cultures by pulse labeling DNA at specific times with 14C-thymidine and following incorporation of radionucleotide into genomic Not I restriction fragments. This technique could be used to characterize chromosomal DNA replication, to characterize mutations which affect this process, to identify the location of DNA replication origins and termini as well as aid in the construction of macrorestriction maps. Here, we further characterize the DNA replication mutations divE and dnaK and preliminary characterize the genomic organization of E. coli isolate 15.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Tomizawa J. Chromosome replication in Escherichia coli K12 mutant affected in the process of DNA initiation. Genetics. 1971 Sep;69(1):1–15. doi: 10.1093/genetics/69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Fujimoto S., Ozaki N. Molecular cloning and nucleotide sequencing of the gene for E. coli cAMP receptor protein. Nucleic Acids Res. 1982 Feb 25;10(4):1345–1361. doi: 10.1093/nar/10.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber W., Wauters-Willems D. Host specificity of DNA produced by Escherichia coli. XII. The two restriction and modification systems of strain 15T-. Mol Gen Genet. 1970;108(3):203–217. doi: 10.1007/BF00283350. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Sekimizu K., Funnell B. E., Kornberg A. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell. 1986 Apr 11;45(1):53–64. doi: 10.1016/0092-8674(86)90537-4. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C., Tilly K., Drahos D., Hendrix R. The B66.0 protein of Escherichia coli is the product of the dnaK+ gene. J Bacteriol. 1982 Mar;149(3):1175–1177. doi: 10.1128/jb.149.3.1175-1177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. B., Atlung T., Hansen F. G., Skovgaard O., von Meyenburg K. Fine structure genetic map and complementation analysis of mutations in the dnaA gene of Escherichia coli. Mol Gen Genet. 1984;196(3):387–396. doi: 10.1007/BF00436184. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Henson J. M., Kuempel P. L. The terminus region of the Escherichia coli chromosome contains two separate loci that exhibit polar inhibition of replication. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1754–1758. doi: 10.1073/pnas.84.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Kopp B. J., Kuempel P. L. Termination of DNA replication in Escherichia coli requires a trans-acting factor. J Bacteriol. 1988 Feb;170(2):662–668. doi: 10.1128/jb.170.2.662-668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark C., Arber W. Host specificity of DNA produced by Escherichia coli. 13. Breakdown of cellular DNA upon growth in ethionine of strains with r plus-15, r plus-P1 or r plus-N3 restriction phenotypes. J Mol Biol. 1970 Sep 14;52(2):337–348. doi: 10.1016/0022-2836(70)90034-3. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Renger H. Initiation of DNA replication in Escherichia coli 15T-: chronological dissection of three physiological processes required for initiation. J Mol Biol. 1969 Jun 14;42(2):221–235. doi: 10.1016/0022-2836(69)90039-4. [DOI] [PubMed] [Google Scholar]
- Liberek K., Georgopoulos C., Zylicz M. Role of the Escherichia coli DnaK and DnaJ heat shock proteins in the initiation of bacteriophage lambda DNA replication. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6632–6636. doi: 10.1073/pnas.85.18.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Messer W., Noyer-Weidner M. Timing and targeting: the biological functions of Dam methylation in E. coli. Cell. 1988 Sep 9;54(6):735–737. doi: 10.1016/s0092-8674(88)90911-7. [DOI] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Ohki M., Sato S. Regulation of expression of lac operon by a novel function essential for cell growth. Nature. 1975 Feb 20;253(5493):654–656. doi: 10.1038/253654a0. [DOI] [PubMed] [Google Scholar]
- Ohki M., Tamura F., Nishimura S., Uchida H. Nucleotide sequence of the Escherichia coli dnaJ gene and purification of the gene product. J Biol Chem. 1986 Feb 5;261(4):1778–1781. [PubMed] [Google Scholar]
- Oki M., Mitsui H. Defective membrane synthesis in an E. coli mutant. Nature. 1974 Nov 1;252(5478):64–66. doi: 10.1038/252064a0. [DOI] [PubMed] [Google Scholar]
- Pyle L. E., Finch L. R. A physical map of the genome of Mycoplasma mycoides subspecies mycoides Y with some functional loci. Nucleic Acids Res. 1988 Jul 11;16(13):6027–6039. doi: 10.1093/nar/16.13.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokeach L. A., Zyskind J. W. RNA terminating within the E. coli origin of replication: stringent regulation and control by DnaA protein. Cell. 1986 Aug 29;46(5):763–771. doi: 10.1016/0092-8674(86)90352-1. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y. The dnaK gene of Escherichia coli functions in initiation of chromosome replication. J Bacteriol. 1988 Feb;170(2):972–979. doi: 10.1128/jb.170.2.972-979.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Ohki M., Yura T., Ito K. Genetic studies of an Escherichia coli K-12 temperature-sensitive mutant defective in membrane protein synthesis. J Bacteriol. 1979 May;138(2):305–313. doi: 10.1128/jb.138.2.305-313.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Saffran W., Welsh J., Haas R., Goldenberg M., Cantor C. R. New techniques for purifying large DNAs and studying their properties and packaging. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):189–195. doi: 10.1101/sqb.1983.047.01.024. [DOI] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Skelly S., Fu C. F., Dalie B., Redfield B., Coleman T., Brot N., Weissbach H. Antibody to sigma 32 cross-reacts with DnaK: association of DnaK protein with Escherichia coli RNA polymerase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5497–5501. doi: 10.1073/pnas.85.15.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Kolodner R. D. Mapping of Escherichia coli chromosomal Tn5 and F insertions by pulsed field gel electrophoresis. Genetics. 1988 Jun;119(2):227–236. doi: 10.1093/genetics/119.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura F., Nishimura S., Ohki M. The E. coli divE mutation, which differentially inhibits synthesis of certain proteins, is in tRNASer1. EMBO J. 1984 May;3(5):1103–1107. doi: 10.1002/j.1460-2075.1984.tb01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung B. Y., Kornberg A. Membrane attachment activates dnaA protein, the initiation protein of chromosome replication in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7202–7205. doi: 10.1073/pnas.85.19.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Massy B., Béjar S., Louarn J., Louarn J. M., Bouché J. P. Inhibition of replication forks exiting the terminus region of the Escherichia coli chromosome occurs at two loci separated by 5 min. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1759–1763. doi: 10.1073/pnas.84.7.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]







