Abstract
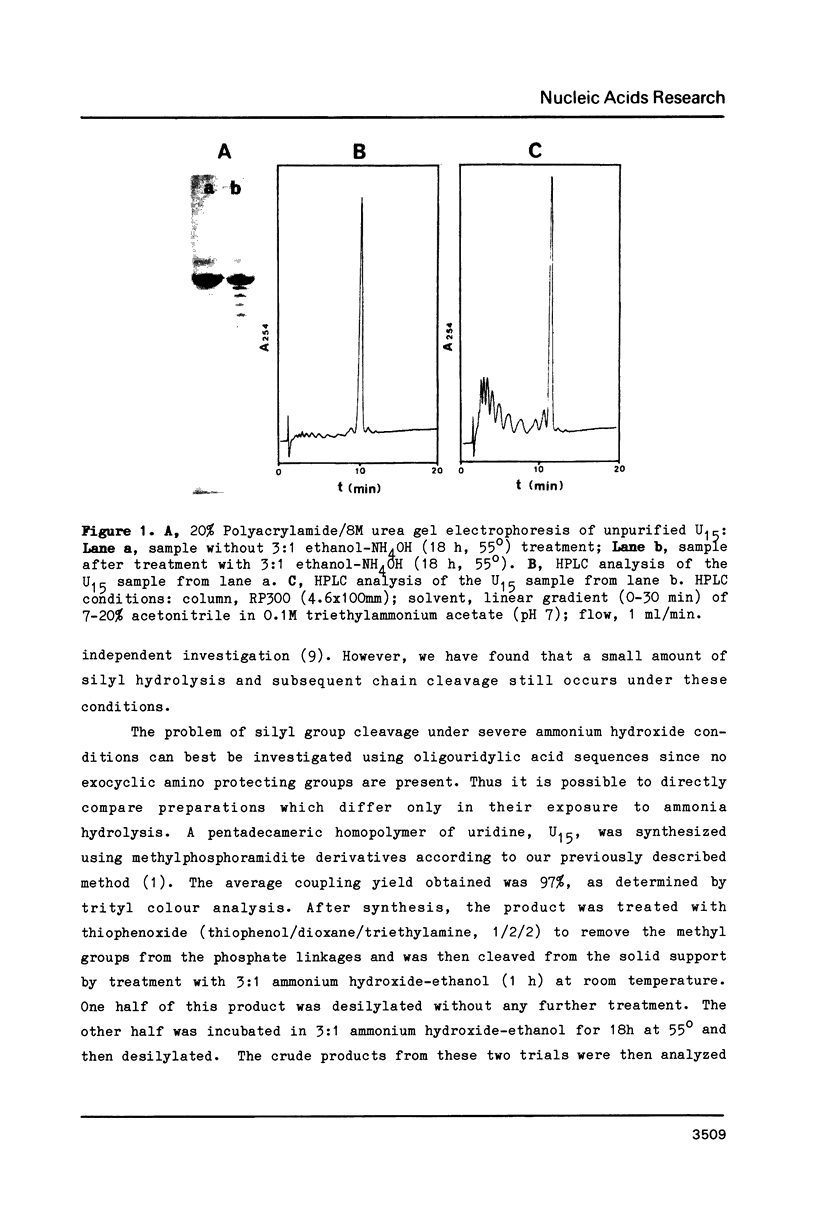
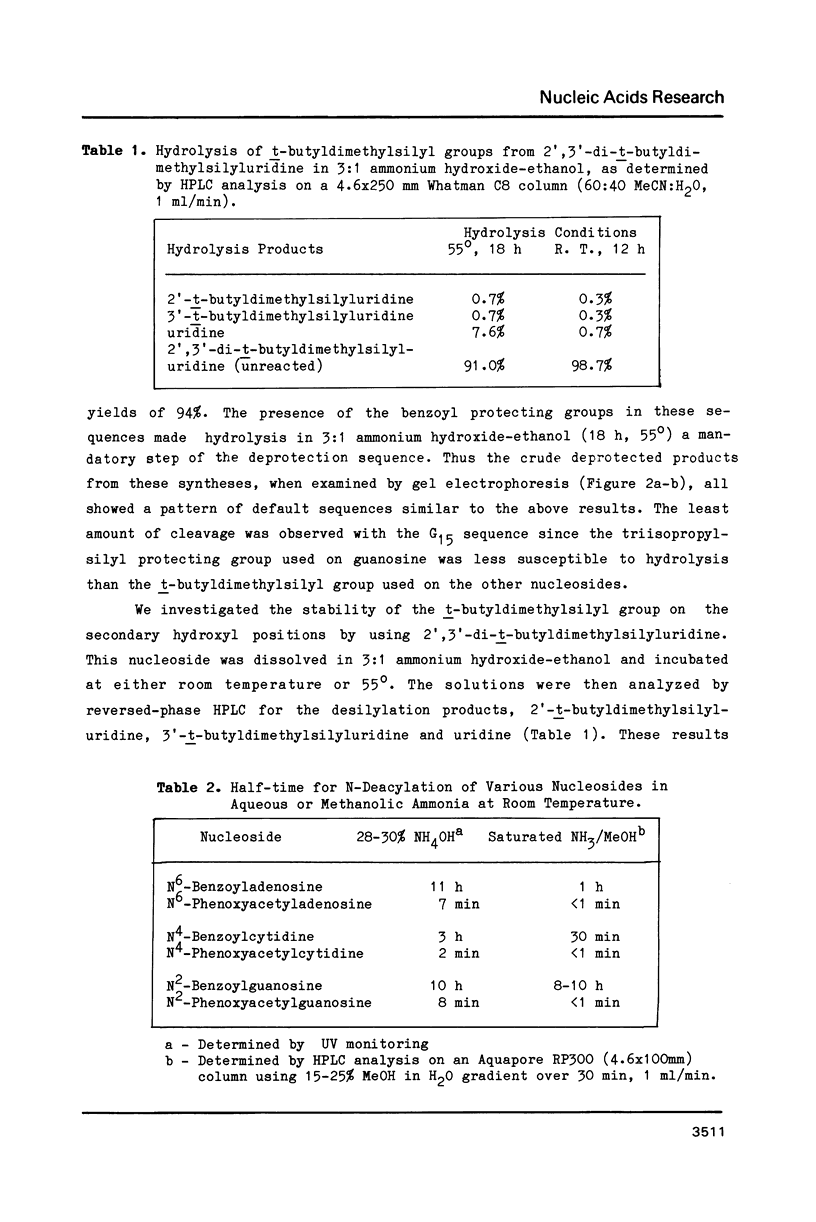
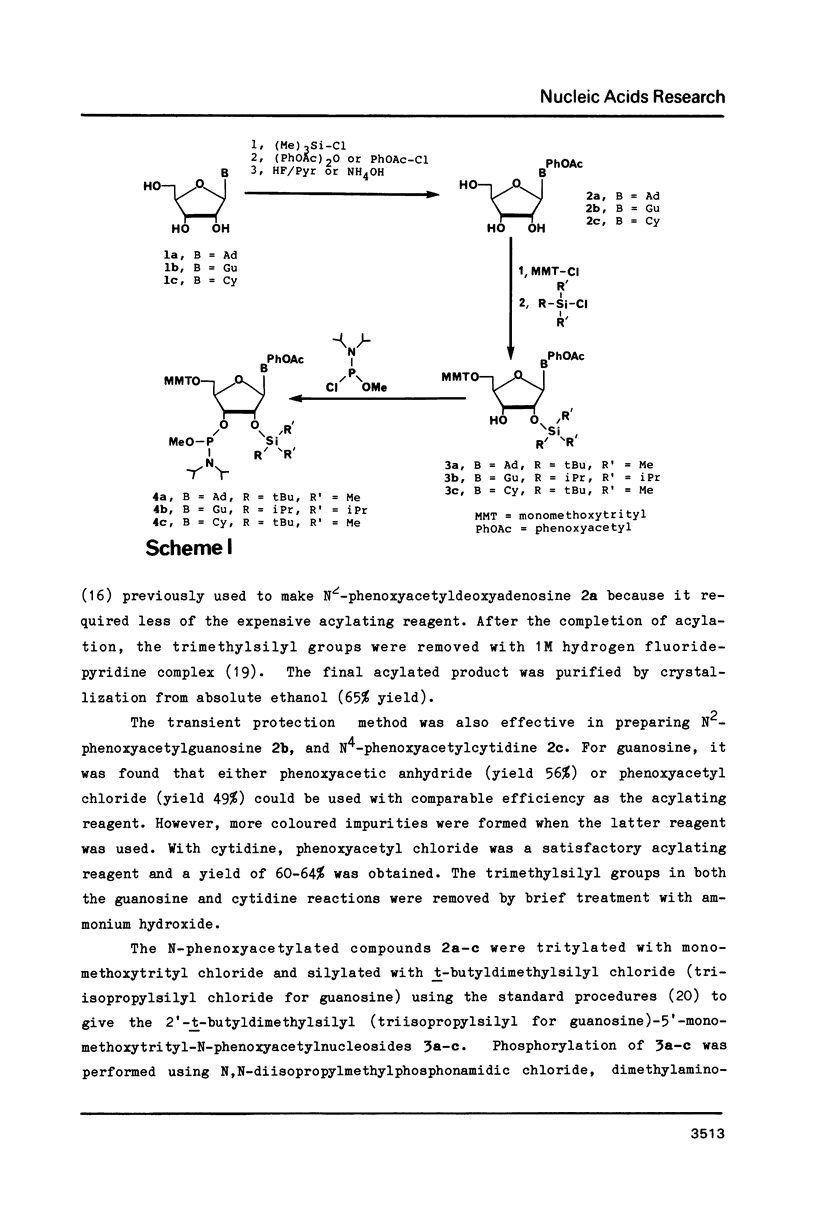
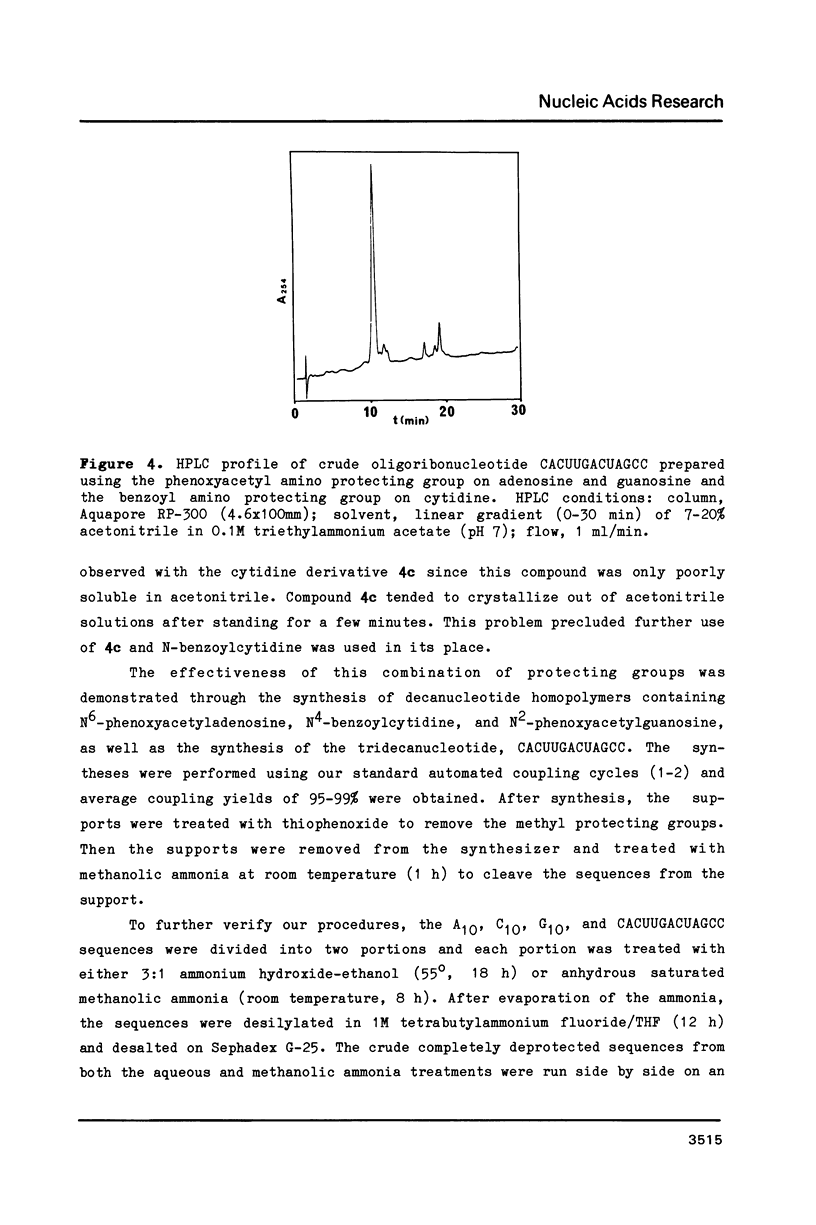
Strong aqueous ammonium hydroxide used to remove N-acyl protecting groups from synthetic oligoribonucleotides causes removal of some alkylsilyl protecting groups from 2'-hydroxyls and leads to chain cleavage. This problem is most severe when 30% ammonium hydroxide is used and substantially reduced but still detectable when 3:1 ammonium hydroxide-ethanol is used. We have virtually eliminated this unwanted cleavage by incorporating the labile phenoxyacetyl amino protecting group on adenosine and guanosine. The N-benzoyl protecting group remains adequate for cytidine nucleosides. Synthetic oligoribonucleotides containing these N-acylated nucleosides and 2'-t-butyldimethylsilyl or 2'-triisopropylsilyl protecting groups can be deacylated by room temperature treatment in saturated anhydrous methanolic ammonia (8-12 h) without causing any detectable chain cleavage.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Itakura K., Rossi J. J., Wallace R. B. Synthesis and use of synthetic oligonucleotides. Annu Rev Biochem. 1984;53:323–356. doi: 10.1146/annurev.bi.53.070184.001543. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Pon R. T. The chemical synthesis of oligoribonucleotides VII. A comparison of condensing agents in the coupling of silylated ribonucleosides. Nucleic Acids Res. 1980 May 10;8(9):2105–2115. doi: 10.1093/nar/8.9.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie K. K., Theriault N., Sadana K. L. Synthesis of oligoribonucleotides. J Am Chem Soc. 1977 Nov 9;99(23):7741–7743. doi: 10.1021/ja00465a073. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulhof J. C., Molko D., Teoule R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987 Jan 26;15(2):397–416. doi: 10.1093/nar/15.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J., Strömberg R., Thelin M., Westman E. Studies on the t-butyldimethylsilyl group as 2'-O-protection in oligoribonucleotide synthesis via the H-phosphonate approach. Nucleic Acids Res. 1988 Oct 11;16(19):9285–9298. doi: 10.1093/nar/16.19.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]