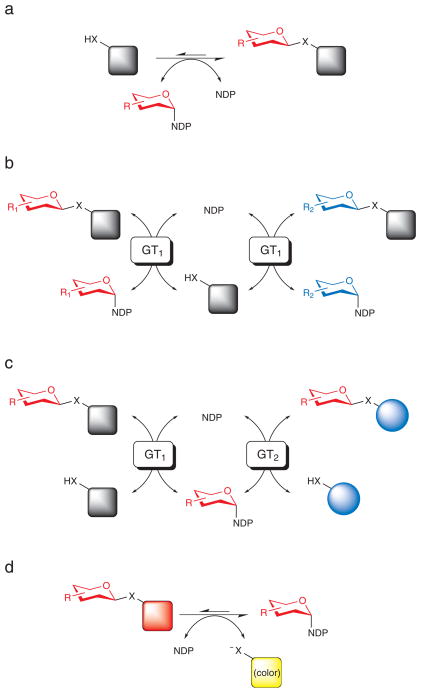
Fig. 1.
Representative GT-catalyzed reactions. a) Classical GT-catalyzed transformation wherein the sugar, presented in the form of a sugar nucleotide donor, is conjugated to an acceptor target of interest to provide a thermodynamically-favored glycoside product. b) A GT-catalyzed ‘sugar exchange’ reaction. In this reaction, a small amount of NDP is used to ‘prime’ the removal of the endogenous sugar appendage of a target complex natural product thus enabling the exchange of a native sugar for an endogenous sugar supplied in vast excess as a sugar nucleotide. c) A GT-catalyzed ‘aglycon exchange’ reaction where the sugar from one complex natural product is excised (using excess NDP) and subsequently attached to a structurally distinct target aglycon. d) The present study demonstrates the use of simple activated glycosides to dramatically shift the thermodynamics of GT-catalyzed reactions and thereby drive GT-catalyzed NDP-sugar synthesis, sugar exchange/and or aglycon exchange reactions while also offering a convenient colorimetric screen for glycosylation.