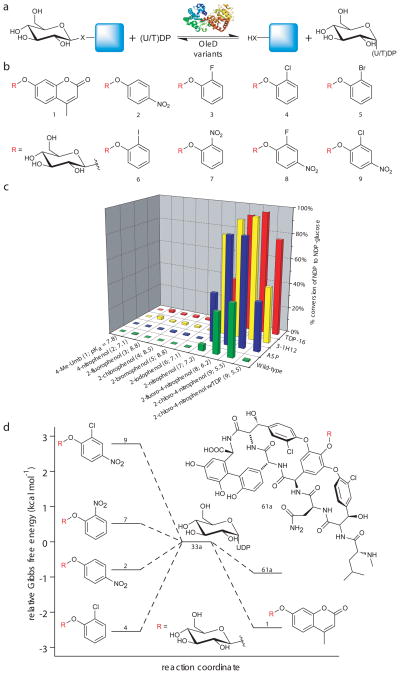
Fig. 2.
Evaluation of putative donors for sugar nucleotide synthesis. (a) General reaction scheme. (b) Structures of the β-D-glucopyranoside donors which led to (U/T)DP-glucose formation. (c) Percent conversion of (U/T)DP to (U/T)DP-glucose with various donors (n ≥ 2, standard deviation ≤ 5%). Reactions contained 2.1 μM (10 μg) OleD variant, 1 mM of (U/T)DP, and 1 mM of aromatic donor (1–9) in Tris-HCl buffer (50 mM, pH 8.5) with a final volume of 100 μl. After one hour at 25°C, reactions were flash frozen and analyzed by HPLC (Supplementary Methods). The pKa for each corresponding donor aglycon is highlighted in parentheses. (d) Plot depicting the relative Gibbs free energy of selected donors/acceptors in relation to 33a. Small glycoside donors display large shifts in relative free energy, transforming formation of UDP-Glc (33a) from an endo- to an exothermic process. The ΔG°pH8.5 for 1, 2, 4, 7, and 9 with UDP in Tris-HCl buffer (50 mM, pH 8.5) at 298K relative to 33a were determined in this study (Supplementary Methods). The ΔG° for 61a was previously determined (at pH 9.0 and 310K)(5).