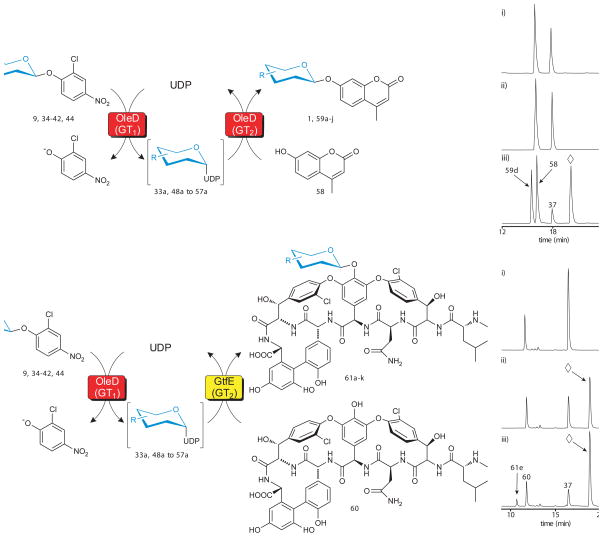
Fig. 4.
Evaluation of 2-chloro-4-nitrophenyl glycosides as sugar donors in coupled GT-catalyzed transglycosylation reactions. (a) The scheme for a single enzyme (TDP-16) coupled system with 4-methylumbelliferone (58) as the final acceptor (left) and a representative HPLC analysis (right) using the donor for 6-azido-6-deoxy-D-glucose (37). Reactions contained 1 mM glycoside donor, 1 mM 58, 1 mM UDP, and 11 μM TDP-16 in a total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) at 25°C for 24 hour and were subsequently analyzed by HPLC (Supplementary Methods). For the representative reaction: (i) control reaction lacking TDP-16; (ii) control reaction lacking UDP; (iii) full reaction where 37 is donor, 58 is acceptor, 59d is desired product and ⋄ represents 2-chloro-4-nitrophenolate. (b) The scheme for a double enzyme (TDP-16 and GtfE) coupled system with vancomycin aglycon (60) as the final acceptor (left) and a representative HPLC analysis (right) using the donor for 6-azido-6-deoxy-D-glucose (37). Reactions contained 1 mM glycoside donor, 0.1 mM 60, 1 mM UDP, 11 μM TDP-16, and 11 μM GtfE in a total volume of 100 μl with Tris-HCl buffer (50 mM, pH 8.5) at 25°C for 24 hour and were subsequently analyzed by HPLC (Supplementary Methods). For the representative reaction: (i) control reaction lacking TDP-16; (ii) control reaction lacking GtfE; (iii) full reaction where 37 is donor, 60 is acceptor, 61e is desired product and ⋄ represents 2-chloro-4-nitrophenolate. Sample preparation and HPLC parameters, along with chromatograms (Supplementary Fig. 14 and 17), conversion rates, and mass characterization (Supplementary Table 4 and 5) for all products are presented in supporting online material.