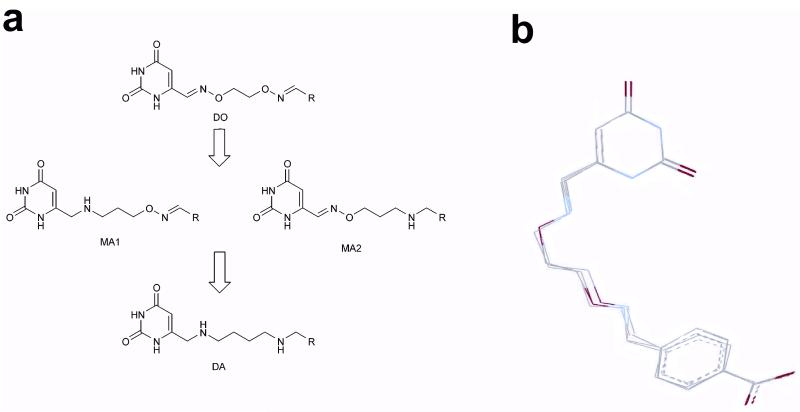
Figure 2.
Diversification of rigid bivalent oxime linkers into flexible monoamine and diamine linkers. (a) The rigid and planar sp2 centers of the dioxime (DO) linkers can be systematically converted into more flexible sp3 linkages using amine chemistry. Thus, the original uracil fragment libraries can be transformed into three different amine libraries. The monoamine (MA) libraries have a flexible sp3 amine center at the uracil end of the tether (MA1), or the diversified end of the tether (MA2). The diamine (DA) library has flexible amine centers at both ends of the tether. (b) The oxime and amine linkers can present the uracil and 4-carboxybenzaldehyde (29) binding fragments in the observed productive binding mode shown in Figure 1b. MMF3 molecular mechanics computations were used to superimpose the corresponding MA1, MA2 and DA linker versions with the crystallographically determined bound conformation of 13. In this computation, the uracil and carboxylate atoms of each compound were superimposed and frozen while the linkers were allowed to equilibrate.