Abstract
Response to stress is determined in part by genetically-influenced regulation of the monoamine system. We examined the interaction of a stressor (receipt of public assistance) and a gene regulating the monoamine system (MAOA) in the prediction of change in adolescent depressive symptoms and body mass index (BMI). Participants were drawn from the National Longitudinal Study on Adolescent Health (AddHealth) genetically-informative subsample. We focused on males due to the fact that males only have one MAOA allele. Growth curve analyses were conducted to assess the association between public assistance, MAOA allele, and their interaction and the intercept and slope of depressive symptoms and BMI. The results indicated that among males, MAOA allele type interacted with receipt of public assistance in the prediction of rate of change in both depressive symptoms and BMI from early adolescence through early adulthood. Males with the short MAOA allele whose families received public assistance tended to experience increased growth in depressive symptoms and BMI. Implications of the findings for understanding the relations among stress, physiology, and development are discussed.
Interactions Between MAOA Genotype and Receipt of Public Assistance: Predicting Change in Depressive Symptoms and Body Mass Index
Adolescents from low-income families are at substantially increased risk for adverse developmental outcomes in areas such as psychological adjustment and health (Ackerman, Kogos, Youngstrom, Schoff, & Izard, 1999; Duncan, Yeung, Brooks-Gunn, & Smith, 1998; Hart, Atkins, & Matsuba, 2008; Korenman, Miller, & Sjaastad, 1995). One explanation for the correlation of family income with developmental outcome is that family impoverishment results in stress, and that stress interferes with adolescent development. Children living in poor families are more likely to experience high levels of stress within their homes (Evans, Gonnella, Marcynyszyn, Gentile, & Salpekar, 2005) and tend to live in neighborhoods characterized by stressors such as crime, noise, and dilapidated housing (Evans, 2004). Physiological indices of stress such as cortisol response and autonomic sensitivity are associated with both poor adjustment (Hart, Eisenberg, & Valiente, 2007) and family income (Lupien, King, Meaney, & McEwen, 2001). Receiving public assistance appears to be a particularly stressful experience for families, due to the combination of this low-income status with other factors that are specifically associated with receiving public assistance (Blank & Ruggles, 1996; Edin & Lein, 1997).
Remarkably, while there is ample evidence to suggest both that adolescents living in poverty experience heightened levels of stress and that stress can affect development, many adolescents from low-income families—including those that receive public assistance—thrive (Werner, 1995). The diversity of outcomes among adolescents living in stressful conditions has led to the search for factors that mediate and moderate the effects of stress on development. In this study, we examine a potential genetic moderator of the association between a stressor (specifically, receipt of public assistance) and the development of adolescent depressive symptoms and body-mass index (BMI) and extend our understanding of the relation between stress and developmental outcome in two interrelated ways. First, we test whether receiving public assistance exhibits the same interaction with a genetic marker as do other stressors such as child abuse. Evidence for similarity of relations with genes would suggest that receiving public asssitance effects its influence on development partly through the same biological mechanisms as do other stressors. Second, evidence for genetic moderation of public assistance on development would contribute to an explanation for the range of outcomes characterizing adolescents living in low-income families.
Associations between Economic Hardship, Stress, and Receipt of Public Assistance
Poverty-related stress can be conceptualized and measured in a number of different ways. For example, the federal poverty line, which involves applying a cut-off based on total family income relative to family size, can be used as a criterion. It is also possible to use perceived need, such as by asking people whether they have needed to forgo essentials due to financial problems. Total family income is another way of conceptualizing potential hardship, although this does not adjust for family size or cost of living. We chose to use receipt of public assistance as our criterion for several reasons. First, it involves a clear statement of need by the family (applying for the assistance), as well as the clear determination of need by local criteria (thereby providing a crude adjustment for cost-of-living differences across regions and family sizes). Second, the process of applying for public assistance typically involves some difficulty and stress, which likely indicates that the families receiving it were experiencing stress related to their economic status. In fact, research has demonstrated that among familes who are eligible for public assistance, families who participate in these programs are often characterized by parents with lower possibilities for current and future earnings and long-term, relative to short-term, periods of eligibility (Blank & Ruggles, 1996). These long-term periods of need, combined with few opportunities for earnings either now or in the future, would likely increase stress in the family. Third, families receiving public assistance also need to deal with the stigma associated with receiving public assistance, and potential shame relating to not being economically self-sufficient. Fourth, previous research has demonstrated that for families receiving public assistance, that assistance is often insufficient to meet the family’s basic needs, which is stressful and compels families to find other “survival strategies” to make ends meet (Edin & Lein, 1997). Finally, using this criterion was suitable from a practical standpoint: approximately 9% of our sample received public assistance, which provided us with a reasonable sample size to analyze.
Stress and the Monoamine System
Physiological response to stress involves multiple systems that affect mood, behavior, and health. Among these is the monoamine system which includes the neurotransmitters norepinephrine, serotonin, and dopamine (Flugge, van Kampen, & Mijnster, 2004). The monoamine system is responsive to short-term stress and its functioning may be permanently disordered by enduring stress (Flugge et al.). Dysregulation of the monoamine system is associated with depression (e.g, Heim, Plotsky, & Nemeroff, 2004) and obesity (e.g., Schwartz, Woods, Porte, Seeley, & Baskin, 2000), findings that together suggest that depression and obesity can result from a single process affected by interaction of stress and genes. Indeed, a recent review concluded that the biological underpinnings of obesity and depression may both relate to dysregulation in the stress-response system (Bornstein, Schuppenies, Wong, & Licinio, 2006). This observation is consistent with the literature indicating that obesity (as well as increases in BMI) is cross-sectionally related to increased risk for depression in community-based samples (e.g., Petry, Barry, Pietrzak, & Wagner, 2008; Simon et al., 2006). In addition, longitudinal data indicates that depression in childhood is associated with increased BMI in adulthood (Pine, Goldstein, Wolk, & Weissman, 2001) and among adults, obesity is associated with an increased risk of depression five years later (Roberts, Deleger, Strawbridge, & Kaplan, 2003). Reviews of the literature have suggested that there may be similar genetic or environmental factors that increase risk for both disorders (e.g., Stunkard, Faith, & Allison, 2003). Thus, due to the associations between these problems and the possibility that similar genetic and/or environmental factors may increase risk for both problems, we examined both BMI and depressive symptoms as outcome variables that represent aspects of the mental and physical health of adolescents and young adults.
Individual differences in the regulation of the monoamine system are associated with polymorphisms in the MAOA gene. This gene produces MAO-A, an enzyme involved in the catabolism of serotonin and other monoamines. Polymorphisms on the MAOA gene affect transcription, with the short version (allele) of the polymorphism producing less MAO-A than the longer allele (Meyer-Lindenberg et al., 2006). Research suggests that the longer allele is associated with more advantageous outcomes following extreme stress such as child abuse (e.g., Caspi et al., 2002; Kim-Cohen et al., 2006), presumably as a result of high levels of MAO-A effectively regulating the monoamine system. Buckholtz et al. (2007) demonstrated that the amygdala and ventromedial prefrontal cortex (both neurological substrates of emotion regulation) interact differently in those with different variants of the MAOA gene, and suggested that those with the short version of the allele might be more prone to maladaptive change in temperament as a result of environmental influence.
Most of this research focuses on males only. Because the MAOA gene is on the X chromosome, males have only one allele, thus simplifying the study of the relation of the allele to mood and emotion (in contrast, females have two alleles and in any heterozygous individual, it is unclear how much MAO-A a given heterozygous female has). Moreover, there is evidence of a MAOA-by-gender interaction. Specifically, Meyer-Lindenberg et al. (2006) conducted MRI scans of healthy humans with different MAOA alleles and found that the low expression (short) variant of this allele was associated with several physiological correlates (e.g., structural change in the orbitofrontal cortex, an area that relates to patterns of learning in response to reinforcers and lesions of which relate to disinhibition and antisocial behavior) among men but not among women. In addition, among primates, MAOA allele type is associated with dominance and aggression in males but not in females (Newman, Syagailo, Barr, Wendland, Champoux, Graessle, Suomi, Higley, & Lesch, 2005). This suggests that MAO levels affect males and females differently. Although the exact mechanism behind these sex differences is unclear, we know that estrogen affects how MAOA is transcribed in the brain (Gundlah, Lu, & Bethea, 2002). Therefore, although research to date has been minimal and the mechanisms of potential sex differences remain unclear, it seems possible that MAOA allele type is more influential in males than in females.
Gene-By-Environment Interactions in Development
Other studies have supported the utility of research examining interactions between particular genes and environmental stressors in the prediction of depression. For example, Caspi et al. (2003) reported that a particular polymorphism in the serotonin transporter gene moderated the association between stressful life events and depression-related outcomes. Similarly, Kaufman et al. (2004) found that the short allele of the serotonin transporter gene moderates risk for depression among maltreated children. Although we are unaware of research specifically examining the interaction of MAOA alleles and stress in the prediction of depression, MAOA has been shown to moderate response to stress in the prediction of emotional problems in general (Kim-Cohen, 2006), and the MAOA genes regulate the catabolism of neurotransmitters such as serotonin (Meyer-Lindenberg et al., 2006), so this link seems plausible. Regarding BMI, we are unaware of studies examining gene-by-environment interactions. However, research on the effects of fruit juice intake on adiposity gain were suggestive of a gene-by-environment interaction (these effects depended on initial weight status; Faith, Dennison, Edmunds, & Stratton, 2006) and recent research has indicated that MAOA genotype may be related to obesity in males (Fuemmeler et al., 2008); therefore, it seems reasonable to expect that MAOA genotype and environmental adversity may interact in the prediction of BMI and/or obesity.
Current Study
We tested for potential interactions of receipt of public assistance with polymorphisms in the MAOA gene in the prediction of longitudinal trajectories of depressive symptoms and BMI. We expected that the receipt of public assistance would interact with the short version of the allele for the MAOA genes to predict increasing depression symptom scores and BMI over time (positive slopes). We expected weaker (non-significant) effects on initial levels of these variables for two reasons: (1) genetic effects may become stronger with increasing age (Bouchard & McGue, 2003); (2) some of the stressful aspects of receiving public assistance are likely to become more stressful with increasing age (as adolescents become aware of societal expectations for families to be economically self-sufficient, as they feel more pressure to fit in with their peers, and as they realize the implications that their family’s status has for their own future [e.g., difficulty paying for college]).
We chose to examine developmental trajectories of depressive symptoms and BMI— instead of outcome at a single age or point in time—for several reasons. First, implicit in the gene-by-environment research reviewed above is the assumption that the interaction of stress with genes changes the developmental trajectories of individuals. However, we are aware of no prior gene-stress interaction research in which adjustment and health were examined over time in order to test for change in developmental trajectories. In this study, multiple measures of depressive symptoms and BMI allowed us to characterize participants’ development through time with growth curves (see Singer & Willett, 2003, for a discussion of the advantages of this approach). Second, there are several disadvantages to examining outcomes at a single point in time. For example, a person may be generally experiencing an increase in depressive symptoms, but may be having a particularly good week at a certain assessment and therefore may report decreased symptoms; including multiple assessment points minimizes this problem. In addition, different processes may be at work at different points in development—so if outcomes at, for example, age 18 were examined, it would remain unclear whether similar processes would be at work in early adulthood. Growth curve analyses eliminate these disadvantages associated with using single outcome points by characterizing trends in change over time across the entire period under study (in this case, early adolescence through early adulthood).
Our study focused on males for several reasons: (1) males only have one copy of the MAOA gene, therefore it is always clear which allele is active, whereas females have two copies (complicating the study of heterozygous individuals); (2) previous research has focused on males, which allowed us to make predictions based on prior research for males, but not for females; and (3) there is some indication that MAOA levels may affect males and females differently, so it may be problematic to simply combine males and females into a single sample. However, despite these factors, we decided to report findings for homozygous females in order to contribute to the sparse research literature concerning MAOA in females. In the absence of clear evidence to the contrary, we predicted that we would find the same public assistance-by-MAOA interaction effect predicting increases in depressive symptoms and BMI over time that we expected to find in males; however, this hypothesis was considered quite tentative due to the paucity of prior theory and research in this area on which to base our predictions.
Method
Participants
Participants were drawn from the sub-sample of participants from the National Longitudinal Study of Adolescent Health (AddHealth) for whom DNA data relevant to our hypotheses were available. The full AddHealth sample was a stratified, random sample of students from high schools in the United States; the high schools were stratified into clusters based on region, urbanicity, size, type, percentage white, percentage black, span of grades, and type of curriculum. In addition, students from one “feeder” middle school per high school were included in this study. This full sample included 20,728 students who were in grades 7–12 during the 1994–95 school year (Time 1; mean age=15.66, SD=1.75). Approximately 1 year later (1996), in-home follow-up interviews (Time 2; mean age=16.22, SD=1.65) were conducted with 71% (n=14,738) of these adolescents, and approximately 6 years after the initial assessment (2001–2002; Time 3; mean age=21.96, SD=1.77), 73% of the participants were interviewed (see AddHealth Biomarker Team, n.d., for details).
A sub-sample of those participating at Time 2 were recruited to provide DNA samples. The 2,612 participants in this sub-sample were full- or half-siblings of another participant (monozygotic twins were eliminated for purposes of the present analyses so that we would not examine the same genotype twice); in some families, these sibling pairs may also have been related (e.g., cousins) to others in the sample. Most households in this sample included two siblings; approximately 4% of families included 3 or more participants. MAOA hypothesis-relevant alleles were found in 903 males and 529 females (see below, under Genotyping, for a description of the selection procedure for females for these exploratory analyses). The sample was approximately 60% white, 23% black/African-American, 2% Native American, 7% Asian/Pacific Islander, and 8% Hispanic.
Measures
Depressive symptoms
At Times 1, 2, and 3, participants used a four-point scale (from “never or rarely” to “most of the time or all the time”) to judge how often in the past week they had experienced nine different symptoms (e.g., “felt depressed”). These items were drawn from the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977). Frequency ratings were summed to form scale scores for each of the three testing times (alphas for Time 1–3, respectively: .79, .79, .80). Mean scores (standard deviations) for males at Times 1, 2, and 3, were, respectively: 10.47 (6.73), 10.33 (6.81), and 7.61 (2.65). For females, they were: 12.12 (8.02), 11.55 (7.76), and 8.22 (3.06). This 9-item scale correlated highly with the full CES-D, which was administered at Time 1 and Time 2 (r=.95 and .96, respectively); we chose to use only these 9 items because they were administered at all three assessment points. The CES-D has previously been shown to have considerable validity (e.g., Rende et al., 2006; Steuber & Banner, 2006); for example, it was strongly associated with diagnoses of major depression in other community-based samples of adolescents (e.g., Prescott et al., 1998).
Body mass index (BMI)
BMI was computed by using participants’ reports of height and weight (Time 1) and study assessments of height and weight (Time 2 and 3). Although it would have been ideal to use the same assessment method at each assessment, study assessments were not available at Time 1. As reported in Goodman, Hinden, and Khandelwal (2000), self-reports of BMI (calculated from self-reported height and weight) tend to correlate strongly with measured BMI (r=.92). BMI represents weight in kilograms divided by height in meters squared. Mean BMIs (standard deviations) for males at Times 1, 2, and 3, were, respectively, 22.62 (4.48), 23.28 (4.61), and 26.42 (5.49). For females, they were 22.20 (4.60), 22.59 (4.81), and 26.37 (6.80). Generally, BMIs of 18.5–24.9 are considered normal, BMIs of 25–29.9 are associated with being overweight, and BMIs of 30 or greater are associated with clinical obesity.
Public assistance
At Time 1, a parent was asked “Are you receiving public assistance, such as welfare?” If he or she responded “Yes,” then the family was considered to be receiving public assistance. This indicator of family financial hardship has shown considerable validity in other studies using this data set (e.g., Felson & Hanie, 2002). Family receipt of public assistance was reported for 8.74% of the participants for whom DNA data were available. This is somewhat less than the percentage of families in the United States who were living in poverty at this time (14.5% and 13.8% in 1994 and 1995, respectively; US Census Bureau). The correlation between receipt of public assistance and parent-reported family income (log-transformed due to skew) was r=−.41 (p<.0001).
Genotyping
DNA was isolated from buccal swabs and analyzed at the Institute for Behavioral Genetics at the University of Colorado. Procedures for the assays of the MAOA polymorphism are presented in detail in Haberstick et al. (2005). These procedures resulted in 5 possible fragment sizes: 291, 321, 336, 351, and 381 base pairs, with 97% of the fragments in the AddHealth sample being either 321 or 351 base pair length (Haberstick et al., 2005). DNA amplification followed established procedures (Anchordoquy, McGeary, Liu, Krauter, & Smolen, 2003) and samples were analyzed using an ABI Prism 3100 Genetic Analyzer. Full details of the genotyping are reported elsewhere (Add Health Biomarker Team, n.d.).
Because the MAOA gene is on the X chromosome, males have one allele and females have two. When females are heterozygous (short/long), it is unclear which is active. Therefore, we conducted our primary analyses on males only. We compared males with the short MAOA allele (321; 42%) to males with the long allele (351). In an effort to add to the minimal literature on MAOA in females, we also conducted exploratory analyses on females who were homozygous for allele length (short-short [18% of the sample] or long-long [38% of the sample]).
Age
The age of each participant was recorded at the time of each assessment (means [standard deviations] for Time 1–3 respectively: 15.66 [1.75], 16.22 [1.65], 21.96 [1.77]).
Statistical analyses
Prior to analyses being conducted, each participant’s score on each measure at each assessment wave was converted to a score at each age he or she was assessed. Each participant contributed data at his or her set of three ages (e.g., ages 12, 13, and 18 for one participant; ages 17, 18, and 23 for another).
First, for descriptive purposes, we conducted Pearson correlations among depressive symptoms (at all 3 time points), BMI (at all 3 time points), allele type, and receipt of public assistance.
Multilevel modeling was used to conduct two sets of analyses, each assessing the effects of the interaction of receipt of public assistance and MAOA genotype on: (1) initial levels and rate of change (slope) in depressive symptoms; and (2) initial levels and rate of change (slope) in BMI. Models were estimated using the PROC MIXED procedure in the Statistical Analysis System (SAS) version 9.1 using full information maximum likelihood estimation. Data were clustered both within person (3 assessments per participant) and within family (as noted above, non-monozygotic twin siblings, half-siblings, and cousins were included). Due to this clustering in the data, we employed a three-level model: level 1 was the three assessments nested within each individual participant, level 2 was the participants nested within families, and level 3 provided an estimate of the effects of the predictor variables (public assistance yes/no and MAOA short/long). These analyses, taken together, provided estimates of the effects of public assistance and MAOA allele type (and their interaction) on the intercepts (initial levels) and slopes (rates of change) of the dependent variables (depressive symptoms and BMI), while accounting for clustering in the data (the nesting of participants within families).
Prior to analyses being conducted, we examined the structure of the level 1 residuals; for both depressive symptoms and BMI, these were close to a normal distribution, as assumed by analyses of this type, based on inspection of histograms and skewness statistics. In addition, we investigated the potential influence of extreme residual values by eliminating the .5% of the sample with the lowest and highest residual values and re-running the primary analyses; the pattern of significant results remained identical. We also examined the structure of the level 2 random effects and found that these also approximated a normal distribution. Our model specified unstructured residual correlations.
Once we had conducted the primary analyses using linear models for depressive symptoms and BMI (because of the complexity of the models and the relatively small sample size), we considered whether models with quadratic slope terms for depressive symptoms and BMI would represent better approximations to the data. Specifically, we compared the fit of these models using fit statistics (the Bayesian Information Criterion [BIC]). BIC scores balance fit with parsimony and indicated that the linear models represented better/more parsimonious models (for males’ depressive symptoms, the final linear model had a BIC score of 13964.0, while including a quadratic term for time along with its interactions with the predictors resulted in a BIC score of 13968.8; for males’ BMI, the final linear model had a BIC score of 13482.6, while including a quadratic term for time along with its interactions with the predictors resulted in a BIC score of 13502.7).
Results
Correlations among depressive symptoms, BMI, allele type, and receipt of public assistance are presented in Table 1. As expected, there was continuity in depressive symptoms and BMI over time (all correlations across the three assessment points were significant for both males and females). Cross-sectional correlations between depressive symptoms and BMI tended to be quite modest (though significant). Correlations between MAOA genotype and depressive symptoms and BMI were all non-significant, except for a small positive correlation between the MAOA short allele and T2 depressive symptoms among males. This lack of association between MAOA genotype and the outcome variables (depressive symptoms and BMI) was consistent with the minimal main effects of MAOA genotype on various developmental outcomes (e.g. Foley et al., 2004; Newman et al., 2005). Receipt of public assistance was associated with depressive symptoms at all three time points among both males and females, and also with BMI at all three time points among females. Receipt of public assistance was not correlated with MAOA genotype among either males or females. This indicated that receiving public assistance was not related to genotype; therefore, our consideration of MAOA-by-public assistance interaction effects was truly a consideration of two independent (potential) risk factors, as opposed to factors that were related to each other.
Table 1.
Correlations among Depressive Symptoms, BMI, Receipt of Public Assistance, and Genotype
| T1dep | T2dep | T3dep | T1BMI | T2BMI | T3BMI | MAO-A | |
|---|---|---|---|---|---|---|---|
| Males: | |||||||
| T2dep | .58*** | ||||||
| T3dep | .14*** | .19*** | |||||
| T1BMI | .03** | .05*** | −.01 | ||||
| T2BMI | .03** | .03** | −.01 | .89*** | |||
| T3BMI | .01 | .02 | −.03* | .74*** | .76*** | ||
| MAO-A | .02 | .06* | .03 | .05 | .04 | .04 | |
| Public Ass’t | .08*** | .07*** | .03* | .01 | .02 | .00 | −.03 |
| Females: | |||||||
| T2dep | .58*** | ||||||
| T3dep | .19*** | .21*** | |||||
| T1BMI | .09*** | .07*** | .04*** | ||||
| T2BMI | .10*** | .07*** | .04** | .88*** | |||
| T3BMI | .08*** | .08*** | .03* | .75*** | .79*** | ||
| MAO-A | .01 | .00 | .01 | .03 | .01 | .01 | |
| Public Ass’t | .09*** | .08*** | .03* | .08*** | .09*** | .07*** | .06 |
p<0.05;
p<.01;
p<.001;
T1dep=Time 1 depression; T2dep=Time 2 depression; T3dep=Time 3 depression; T1BMI=Time 1 BMI; T2BMI=Time 2 BMI; T3BMI=Time 3 BMI; MAO-A=MAO risk allele; Public Ass’t=receipt of public assistance at Time 1.
Generally, the results of our growth curve models were consistent with expectations. Allele type moderated the effects of receiving public assistance on adolescent males’ developmental trajectories for depressive symptoms and BMI. Parameter estimates are presented in Table 2. We discuss evidence for moderation for each outcome separately.
Table 2.
Multilevel Model Predicting Depressive Symptoms and BMI from MAOA Genotypes and Receipt of Public Assistance
| Depressive Symptoms | BMI | |||
|---|---|---|---|---|
| Predictor | B (SE) | t | B (SE) | t |
| Males: | ||||
| Age (slope) | −.13 (.03) | −5.20*** | .63 (.02) | 26.24*** |
| Public assistance | 1.07 (.78) | 1.36 | 1.14 (.76) | 1.50 |
| MAOA contrast | .26 (.37) | .70 | .50 (.35) | 1.43 |
| Public assistance * S | −.76 (1.37) | −.55 | −1.76 (1.30) | −1.35 |
| Public assistance * age | −.12 (.09) | −1.42 | −.14 (.08) | −1.67 |
| MAOA contrast * age | −.02 (.04) | −.50 | −.01 (.04) | −.28 |
| Public assistance * S * age | .31 (.15) | 2.06* | .42 (.14) | 2.95** |
| Females: | ||||
| Age (slope) | −.12 (.04) | −2.89** | .69 (.04) | 18.28*** |
| Public assistance | 3.18 (1.07) | 2.98** | .01 (.91) | .01 |
| MAOA contrast S/S | 1.00 (.65) | 1.53 | −.17 (.54) | −.32 |
| Public assistance * S/S | −5.39 (1.76) | −3.06** | .05 (1.5) | .03 |
| Public assistance * age | −.36 (.13) | −2.86** | .14 (.12) | 1.16 |
| MAOA contrast * age | −.12 (.08) | −1.65 | .02 (.07) | .32 |
| Public assistance * S/S * age | .69 (.21) | 3.30** | −.20 (.19) | −1.06 |
p<0.05;
p<.01;
p<.001;
BMI=body mass index; S=short MAOA allele (for contrasts with a long allele in males); S/S=short/short alleles (for contrasts with homozygous long/long alleles in females)
Depressive Symptoms
Random effect results indicated that among males, there was significant variability in both the intercept and the slope of depressive symptoms (z-scores>4.47, p<.0001 for both), indicating that there was variability between people to be explained. The standardized covariance of the intercepts and slopes was −.70, indicating that the higher the depressive symptom score that a boy started with, the more negative his slope in depressive symptoms tended to be.
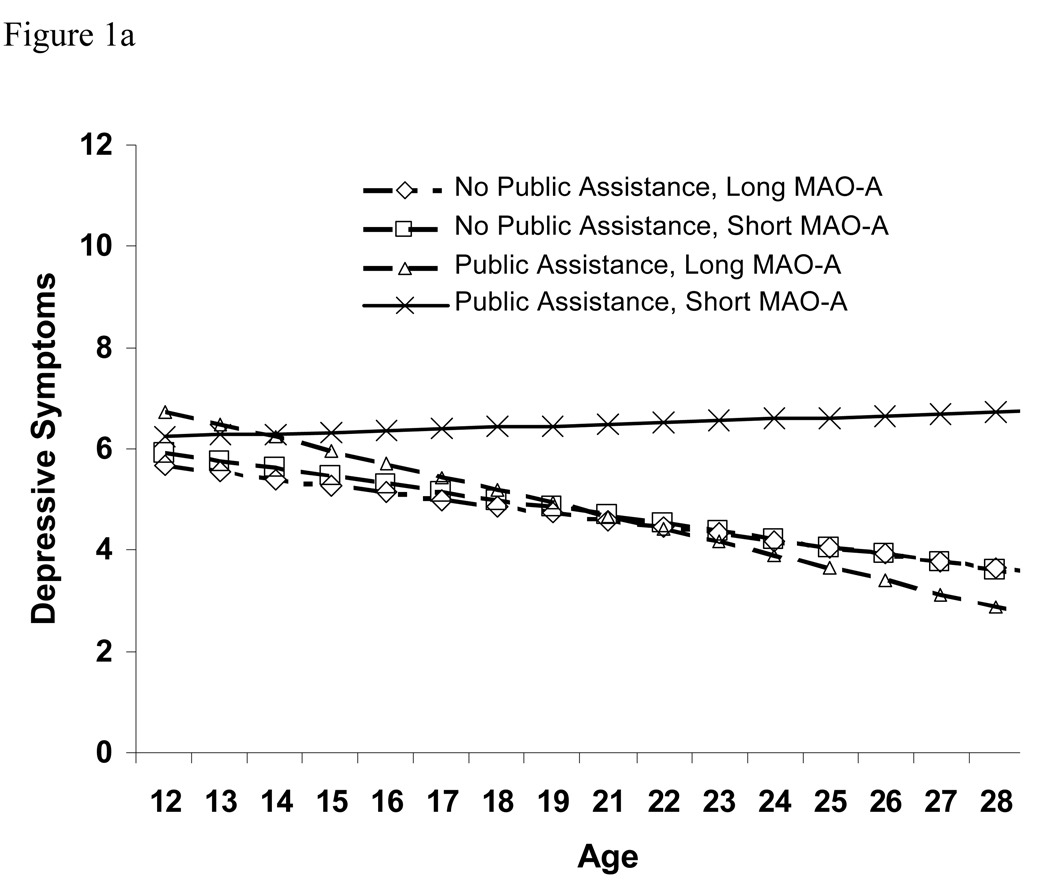
Fixed effect results indicated that among males, the relation of the receipt of public assistance with initial estimates of depressive symptoms (intercept) was not moderated by the MAOA allele. However, consistent with our hypothesis, the interaction of receipt of public assistance and MAOA genotype predicted the rate of increase in depressive symptoms over time—that is, at each point in development, having both of these risk factors was associated with an upward deflection in the slope of depressive symptoms. Figure 1a illustrates this effect: although model-predicted levels of depressive symptoms were similar initially, most groups decreased slowly over time while those with both this MAOA genotype and public assistance increased slightly over time.
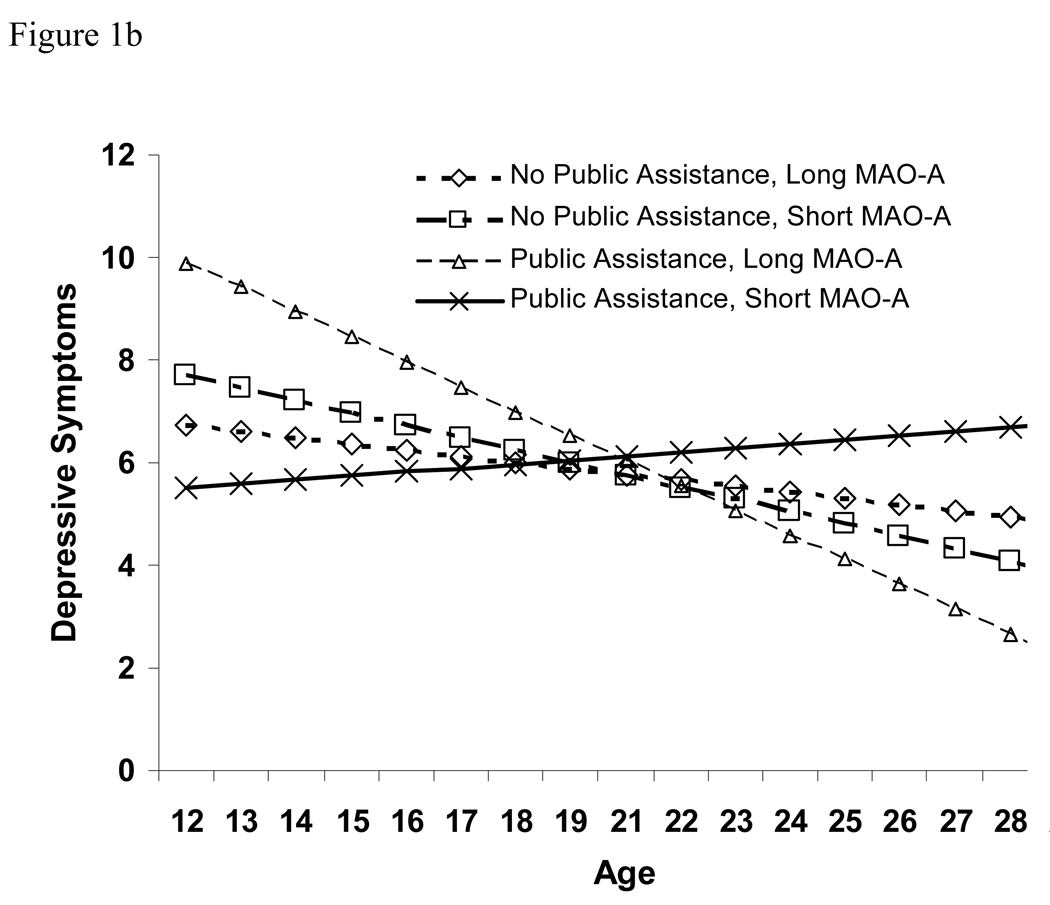
Figure 1.
(a) Estimated developmental trajectories of depressive symptoms for males, by allele type and family receipt of public assistance. Sample sizes in the 4 groups were: 553 long MAO-A, no public assistance; 391 short MAO-A, no public assistance; 51 long MAO-A, public assistance; 28 short MAO-A, public assistance. (b) Estimated developmental trajectories of depressive symptoms for females, by allele type and family receipt of public assistance. Sample sizes in the 4 groups were: 799 long MAO-A, no public assistance; 158 short MAO-A, no public assistance; 78 long MAO-A, public assistance; 25 short MAO-A, public assistance.
Our exploratory analyses examining homozygous females indicated that the patterns of association were fairly similar across males and females. There was significant variability in both the intercept and the slope of depressive symptoms (z-scores>4.87, p<.0001 for both), indicating that there was variability between people to be explained. The standardized covariance of the intercepts and slopes was −.73, indicating that the higher the depression score that a girl started with, the more negative her slope in depressive symptoms tended to be. Fixed effect results indicated that among females, the MAOA allele and receipt of public assistance interacted to predict change in depressive symptoms over time. Figure 1b illustrates this effect: model-predicted levels of depressive symptoms gradually increased for those females with both the MAOA-risk genotype and public assistance, while they decreased for all other groups. However, initial levels of depressive symptoms were somewhat lower among females with both the MAOA-risk genotype and public assistance.
BMI
Random effect results indicated that among males, there was significant variability in both the intercept and the slope of BMI (z-scores>10.41, p<.0001 for both), indicating that there was variability between people to be explained. The standardized covariance of the intercepts and slopes was −.17, indicating that the higher the BMI a boy started with, the less positive his slope in BMI tended to be.
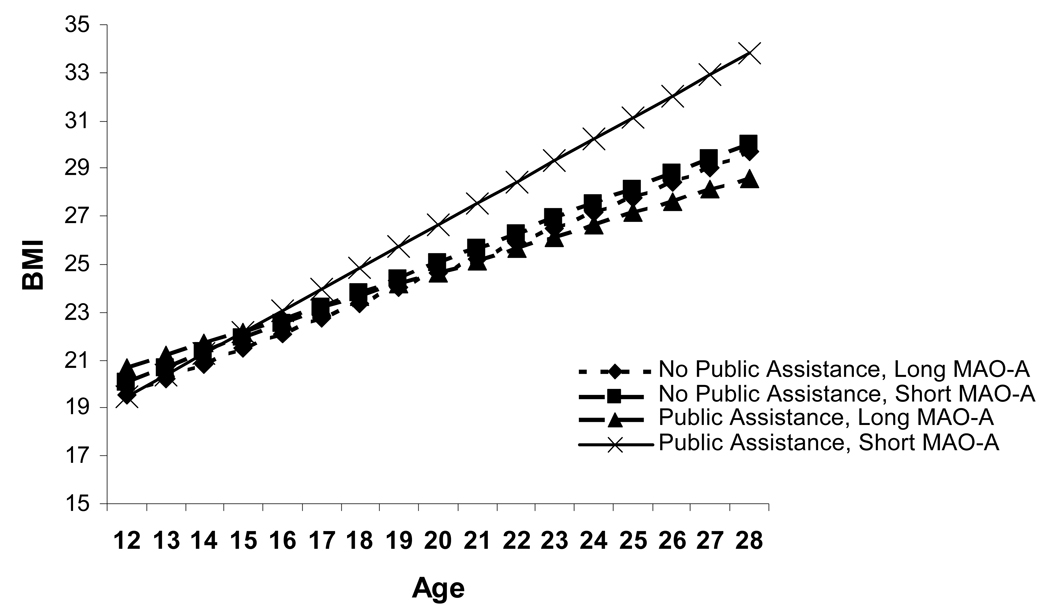
Fixed effect results indicated that among males, findings for BMI mirrored those for depressive symptoms (Table 2). Receipt of public assistance did not interact with MAOA genotype to predict initial levels of BMI, but consistent with our hypothesis, there was a significant interaction of MAOA genotype and receipt of public assistance such that those boys whose families received public assistance and who had the MAOA risk allele increased in BMI most quickly (i.e., having both of these risk factors was associated with an upward deflection in the slope of BMI). This effect is illustrated in Figure 2, and the model-predicted values suggest that the average male from a household receiving public assistance who has the short allele for MAOA is likely to have a BMI in the clinically obese range by early adulthood. The model-predicted BMI for males with the long allele or from households not receiving public assistance is below the cutoff score for clinical obesity for all ages.
Figure 2.
Estimated developmental trajectories of BMI for males, by allele type and family receipt of public assistance. Sample sizes in the 4 groups were: 553 long MAO-A, no public assistance; 391 short MAO-A, no public assistance; 51 long MAO-A, public assistance; 28 short MAO-A, public assistance.
Our exploratory analyses examining homozygous females showed that there was significant variability in both the intercept and the slope of BMI (z-scores>8.83, p<.0001 for both), indicating that there was variability between people to be explained. The standardized covariance of the intercepts and slopes was −.11, indicating that the higher the BMI that a girl started with, the slower her growth in BMI tended to be. However, there were no significant associations between MAOA genotype and receipt of public assistance (or their interaction) and initial levels or rate of change in BMI.
Associations Between Growth in Depressive Symptoms and Growth in BMI
Due to the apparent link between depression and obesity shown by other studies, we also examined Pearson correlations between the slope of depressive symptoms and the slope of BMI. For males, the correlation was r=.04 (p=.08), and for females, the correlation was r=.12 (p<.001). Thus, the associations between rate of change of depressive symptoms and rate of change of BMI were modest, with them being statistically significant for females but not for males.
Discussion
Our goal was to examine whether the developmental trajectories of depressive symptoms and BMI from early adolescence through early adulthood were associated with the interaction of receipt of public assistance and functional polymorphisms in the MAOA gene. This study is the first to demonstrate that males’ developmental trajectories of depressive symptoms and BMI are associated with the interaction of genes and receipt of public assistance. Our findings of a significant gene-by-environment interaction in the prediction of adverse developmental outcomes resemble those obtained by others in the study of the interaction of child abuse and genes (e.g., Caspi et al., 2003). These results suggest that the detrimental effects of growing up in households receiving public assistance on developmental outcome are related, in part, to the differing genetic make-ups of individuals.
Our findings contribute to an explanation for the diversity of outcomes evident in adolescents living in households experiencing economic hardship (Werner, 1995), some of whom receive public assistance. Our results suggest that as a consequence of genetic makeup, some boys who reside in these stressful environments are at increased risk for heightened depressive symptoms and obesity. Specifically, males with short alleles on the MAOA gene living in households receiving public assistance tend to experience an increase in depressive symptoms (when most boys experience a decrease in these symptoms) and an unusually steep increase in BMI during the period from early adolescence through early adulthood.
The physiological and psychological mechanisms that connect genetic variations and stress to the experience of depressive symptoms and the development of obesity are incompletely understood, though progress is rapidly being made in identifying links among genes, biochemical processes, brain structures, and experience (e.g., Meyer-Lindenberg et al., 2006). In addition, recent research has indicated that links between depression and obesity may be partially accounted for by abnormal physiological responses to stress (Bornstein et al., 2006); this makes our finding of similar patterns of MAOA-public assistance interactions in the prediction of both depressive symptoms and BMI among males particularly compelling.
It is important to remember that causality was not determined in this study; that is, although the interaction of receipt of public assistance and MAO genotype was associated with change in depressive symptoms and BMI, these factors may not have caused the changes. There are a myriad of other factors, some associated with our variables and some not, that may have resulted in these changes. Considering some factors that may be associated with our variables, receipt of public assistance is associated with living in a dangerous neighborhood, living in a single-parent household, and attending a low-performing school (Hart & Marmorstein, 2009), any of which could cause stress and contribute to risk for depression and health problems. Similarly, MAOA genotype regulates the catabolism of other neurotransmitters, such as serotonin, norepinephrine, and dopamine; any one (or more than one) of these could actually be responsible for the effects we found. In addition, there are many other risk factors for both depressive symptoms and obesity that we did not examine; this study certainly has not comprehensively explained the development of depression or obesity in the population.
Our exploratory analyses examining the associations between homozygous short MAOA alleles and developmental trajectories of depressive symptoms and BMI in females are the first reported analyses of this sort that we are aware of. These exploratory analyses indicated that for depressive symptoms, the pattern of results was similar as that for males: the combination of living in a household receiving public assistance and having MAOA risk alleles was associated with a faster increase in depressive symptoms over time. However, some differences from males were found. First, model-predicted initial levels of depressive symptoms were actually slightly higher among girls receiving public assistance who did not have the MAOA risk alleles; this resulted in a relatively steep declining slope in model-predicted levels of depressive symptoms among the group who received public assistance but had homozygous long MAOA alleles. Second, no significant MAOA-public assistance interaction was found for the prediction of BMI. In fact, none of the main or interactive effects examined predicted either the intercept or rate of change in BMI among females, indicating that receipt of public assistance and MAOA alleles are not significantly related to BMI in females. Therefore, this study is consistent with other literature that documents somewhat different predictors of obesity in males and females (e.g., Crossman, Sullivan, & Benin, 2006). Although we are unable to explain these differences, we hope that our reporting of these findings will encourage other researchers with relevant data to examine MAOA-stress interactions in girls.
This study contributes to the overall literature on gene-by-environment interactions in several concrete ways. First, it is the first study that we are aware of to examine trajectories of the dependent variables—to specifically examine the interaction of genes and environment on intercepts and slopes (rate of change) of dependent variables. Second, many studies to date have examined severe stressors, such as child abuse; we examined a more common yet less extreme stressor and found that gene-by-environment interactions were still significant. Third, we hope that our exploratory analyses of females can be used by other researchers both to form hypotheses about MAOA in females and to demonstrate how females—albeit only homozygous ones—can be analyzed in a study of this sort.
Of course, there were limitations of this study that should be noted. We assessed depressive symptoms, and not depressive episodes; although not a limitation per se, it should be noted that it is unclear how the present results would apply to people with clinically significant depressive syndromes. It is also unclear whether a given depressive symptom score has the same meaning at different ages; although the CES-D is correlated with major depression in the age groups examined in this study, the specific meaning of different symptoms may differ for younger and older participants. In addition, the assessment of depressive symptoms only inquired about symptoms that may have occurred within the past week. Thus, participants may have had depressive symptoms between the assessment times that were not captured by this assessment. Our assessments of height and weight were not the same at all assessment points; specifically, due to how the AddHealth data were collected, we used self-reports at one time point and study measurements at two other time points. Although participants’ reports correlate strongly with measured BMI (Goodman et al., 2000), this may have affected the results. Finally, it should be noted that this study was not able to establish causal associations between the variables that we studied. Specifically, although we were able to examine how our independent variables—MAOA allele type and receipt of public assistance—were associated with change in depressive symptoms and BMI, clearly the influences on depression and BMI are numerous. Our findings do not indicate that MAOA allele type and/or public assistance cause depression or obesity.
It should also be noted that our choice of receipt of public assistance as our stress-related independent variable may limit the generalizability of these results. For example, many families who experience economic hardship and/or live in poverty do not receive public assistance, and this happens for a multitude of reasons (e.g., racial/ethnic differences in selection into systems of public assistance; different criteria for receiving public assistance according to year, state of residence, etc.; choices regarding whether to apply or not, which appear to be related to factors such as length of the period of eligibility and opportunities for current and future earnings [Blank & Ruggles, 1996]; differential access to application assistance). Therefore, our findings may or may not apply to families living in poverty (and/or experiencing temporary economic hardship) in general. Future research addressing this issue would be helpful.
Our finding that changes in depressive symptoms and BMI are predicted by the interaction of genetic and environmental factors has important implications for our understanding of how both psychopathology and health problems such as obesity develop. Specifically, our results support the notion that obesity and depression may result from pathways shaped by the interaction of chronic stress (in this case, receipt of public assistance) and genes (Bornstein, Schuppenies, Wong, & Licinio, 2006). Therefore, genetic factors alone do not determine risk for these problems, nor do environmental factors alone. Instead, the development of depression and/or obesity under conditions of genetic or environmental risk appears to be related to the presence or absence of the other type of risk factor. Thus, emotional and physical resilience in the presence of risk is not arbitrary—instead, it relates to the presence or absence of other risk factors, which may be operating in different contexts of development.
Acknowledgements
Work on this project was partially supported by a grant from the National Institute on Drug Abuse (K01DA022456). The authors contributed equally to this project. This research uses data from Add Health, a program project designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris, and funded by a grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 17 other agencies. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Persons interested in obtaining data files from Add Health should contact Add Health, Carolina Population Center, 123 W. Franklin Street, Chapel Hill, NC 27516-2524 (addhealth@unc.edu). No direct support was received from grant P01-HD31921 for this analysis.
References
- Ackerman BP, Kogos J, Youngstrom E, Schoff K, Izard C. Family instability and the problem behaviors of children from economically disadvantaged families. Developmental Psychology. 1999;35:258–268. doi: 10.1037//0012-1649.35.1.258. [DOI] [PubMed] [Google Scholar]
- ADD Health Biomarker Team. (n.d.) [Downloaded March 13, 2007];Biomarkers in Wave III of the Add Health Study. from: http://www.cpc.unc.edu/projects/addhealth/files/biomark.pdf.
- Anchordoquy HC, McGeary C, Liu L, Krauter KS, Smolen A. Genotyping of three candidate genes after whole-genome preamplification of DNA collected from buccal cells. Behavior Genetics. 2003;33:73–78. doi: 10.1023/a:1021007701808. [DOI] [PubMed] [Google Scholar]
- Blank RM, Ruggles P. When do women use aid to families with dependent children and food stamps? The dynamics of eligibility versus participation. Journal of Human Resources. 1996;31:57–89. [Google Scholar]
- Bornstein SR, Schuppenies A, Wong M-L, Licinio J. Approaching the shared biology of obesity and depression: The stress axis as the locus of gene-environment interactions. Molecular Psychiatry. 2006;11:892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, McGue M. Genetic and environmental influences on human psychological differences. Journal of Neurobiology. 2003;54:4–45. doi: 10.1002/neu.10160. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Callicott JH, Kolachana B, Hariri AR, Goldberg TE, Genderson M, et al. Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Molecular Psychiatry. 2008;13:313–324. doi: 10.1038/sj.mp.4002020. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moiffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Pulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Crossman A, Sullivan DA, Benin M. The family environment and American adolescents’ risk of obesity as young adults. Social Science and Medicine. 2006;63:2255–2267. doi: 10.1016/j.socscimed.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? American Sociological Review. 1998;63:406–423. [Google Scholar]
- Edin K, Lein L. Work, welfare, and single mothers’ economic survival strategies. American Sociological Review. 1997;62:253–266. [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans GW, Gonnella C, Marcynyszyn LA, Gentile L, Salpekar N. The role of chaos in poverty and children’s socioemotional adjustment. Psychological Science. 2005;16:560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Faith MS, Dennison BA, Edmunds LS, Stratton HH. Fruit juice intake predicts increased adiposity gain in children from low-income families: Weight status-by-environment interaction. Pediatrics. 2006;118:2066–2075. doi: 10.1542/peds.2006-1117. [DOI] [PubMed] [Google Scholar]
- Felson RB, Haynie DL. Pubertal development, social factors, and delinquency among adolescent boys. Criminology. 2002;40:967–988. [Google Scholar]
- Foley D, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Agurs-Collins TD, McClernon FJ, Kollins SH, Kail ME, Bergen AW, Ashley-Koch AE. Genes implicated in serotonergic and dopaminergic functioning predict BMI categories. Obesity. 2008;16:348–355. doi: 10.1038/oby.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E, Hinden BR, Khandelwal S. Accuracy of teen and parental reports of obesity and body mass index. Pediatrics. 2000;106:52–58. doi: 10.1542/peds.106.1.52. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology. 2002;160:271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Haberstick BC, Lessem JM, Hopfer CJ, Smolen A, Ehringer MA, Timberlake D, et al. Monoamine oxidase A and antisocial behaviors in the presence of childhood and adolescence maltreatment. American Journal of Medical Genetics. 2005;1:59–64. doi: 10.1002/ajmg.b.30176. [DOI] [PubMed] [Google Scholar]
- Hart D, Atkins R, Matsuba MK. The association of neighborhood poverty with personality change in childhood. Journal of Personality and Social Psychology. 2008;94:1048–1061. doi: 10.1037/0022-3514.94.6.1048. [DOI] [PubMed] [Google Scholar]
- Hart D, Eisenberg N, Valiente C. Personality change in childhood is predicted by the interaction of family risk with autonomic arousal under stress. Psychological Science. 2007;18:492–497. doi: 10.1111/j.1467-9280.2007.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart D, Marmorstein NR. Neighborhoods and genes and everything in between: Understanding adolescent aggression in social and biological contexts. Development and Psychopathology. 2009;21:961–973. doi: 10.1017/S0954579409000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29:641–648. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: New evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Korenman S, Miller JE, Sjaastad JE. Long-term poverty and child development in the United States: Results from the NLSY. Children and Youth Services Review. 1995;17:127–155. [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic levels. Developmental Psychopathology. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biological Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosomatic Medicine. 2008;70:288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107:1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- Prescott CA, McArdle JJ, Hishinuma ES, Johnson RC, Miyamoto RH, Andrade NN, Edman JL, Makini GK, Nahulu LB, Yuen NYC, Carlton BS. Prediction of major depression and dysthymia from CES-D scores among ethnic minority adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1998;37:495–503. doi: 10.1097/00004583-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rende R, Slomkowski C, Lloyd-Richardson E, Stroud L, Niarua R. Estimating genetic and environmental influences on depressive symptoms in adolescence: Differing effects on higher and lower levels of symptoms. Journal of Clinical Child and Adolescent Psychology. 2006;35:237–243. doi: 10.1207/s15374424jccp3502_7. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: Evidence from the Alameda County Study. International Journal of Obesity. 2003;27:514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Simon GE, von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Archives of General Psychiatry. 2006;63:824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Steuber TL, Banner F. Adolescent smoking and depression: Which comes first? Addictive Behaviors. 2006;31:133–136. doi: 10.1016/j.addbeh.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biological Psychiatry. 2003;54:330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. www.census2010.gov/hhes/www/poverty/histpov/hstpov2.html.
- Werner E. Resilience in development. Current Directions in Psychological Science. 1995;4:81–85. [Google Scholar]