Figure 4.
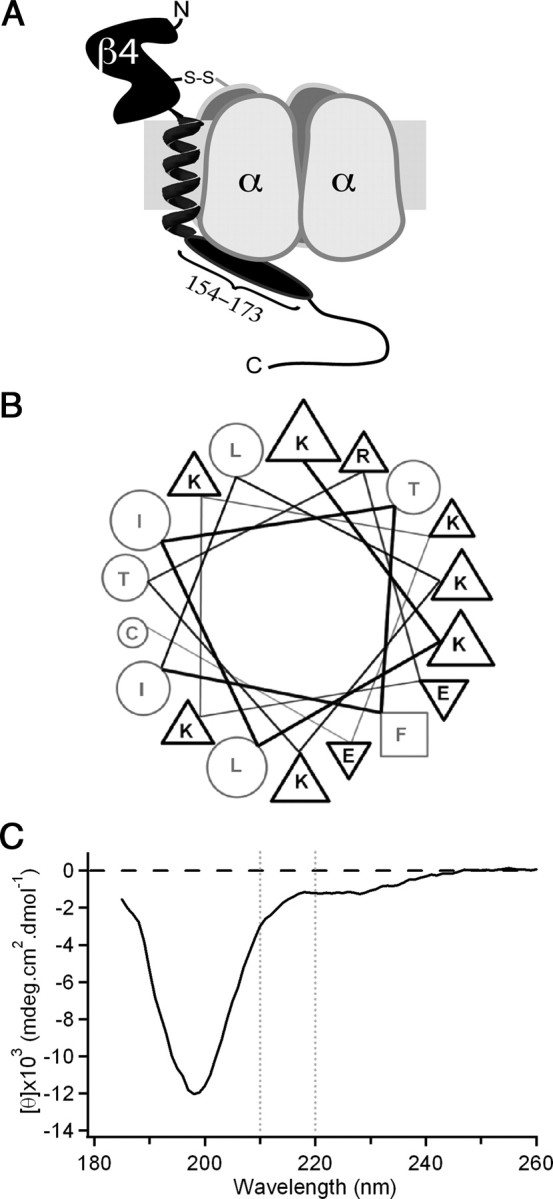
Possible structural conformation of the intracellular tail of NaVβ4. A, Illustration of NaVβ4 (black) covalently attached to a Na channel α subunit (gray). NaVβ4 consists of a large extracellular immunoglobulin-like domain, a single transmembrane helix, and a cytoplasmic tail, the beginning of which comprises the β4 peptide (oval). B, Diagram of mouse extended β4 peptide as an idealized α-helix positions the majority of the charged residues on the same face as the aromatic phenylalanine residue (square). Triangles indicate basic (up) or acidic (down) residues; circles indicate uncharged residues. C, CD spectrum of extended mouse β4 peptide indicates that the sequence is largely unstructured (large minimum at ∼200 nm) with a slight helical propensity (small minimum near 222 nm).
