Abstract
The ability to analyze cryopreserved peripheral blood mononuclear cells (PBMCs) from biobanks for antigen-specific T-cell immunity is necessary to evaluate responses to immune-based therapies. Comprehensive studies have demonstrated that the quality of frozen PBMCs is critical and the maintenance of cell viability and functionality by using appropriate cryopreservation techniques is a key to the successful outcome of assays using PBMCs. Different cryomedia additives affect cell viability. The most common additive is fetal calf serum (FCS), although it is widely known that each FCS lot has to be tested before usage to prevent nonspecific stimulation of T-cells. Also, shipping of samples containing FCS is critical because of many import restrictions. Often, dimethyl sulfoxide (DMSO) is added as a cryoprotectant. However, DMSO concentration has to be reduced significantly because of its toxic effect on cells at room temperature. Therefore, we have developed freezing approaches to minimize cytotoxicity of cryoprotectants and maintain T-cell functionality. We compared different additives to the widely used FCS and found bovine serum albumin fraction V to be an appropriate substitute for the potentially immune-modulating FCS. We also found that DMSO concentration can be reduced by the addition of hydroxyethyl starch. Using our serum-free cryomedia, the PBMC recovery was more than 83% and the PBMC viability was more than 98%. Also, the T-cell functionality measured by enzyme-linked immunospot (ELISpot) was optimal after cryopreservation with our new cryomedia. On the basis of our experimental results, we could finally design 2 different, fully working cryomedia that are standardized, serum free, and manufactured under GMP conditions.
Introduction
Cryopreservation of peripheral blood mononuclear cells (PBMCs) is commonly used to preserve cells for prospective phenotypic and functional analyses in a wide range of infectious disease and clinical vaccine studies. The availability of large repositories of banked specimens allows comparative multicenter studies and avoids inter- and intralaboratory assay variability during analysis of independently isolated fresh samples.1 In the context of HIV vaccine and pathogenesis studies, repositories of frozen PBMC samples allow retrospective monitoring of cellular immune responses by ex vivo assays such as enzyme-linked immunospot (ELISpot). Besides, an increasing number of experimental vaccines are being developed for diseases in which cellular immunity is likely to be required for protection,2 including cancer3 and malaria.4 Accurate quantification of cellular immune responses is important in such studies because they play an essential role in control of viral replication.5,6 The degree to which vaccines induce T-cell responses represents their immunogenic capacity to initiate antibody synthesis. This can be used to compare different vaccine candidates.
Although cryopreservation is a useful tool, it is an extreme process for cells and may result in alterations of cell phenotype and functionality.7 It is known that freezing and thawing can negatively impact functional responses,8–11 particularly to nominal antigens. Cryopreservation can affect antigen processing capability and cause a disproportionate loss of responses to protein antigens.12 There is also a relationship between postthawing viability and the capacity for functional responses.13 Further, the method of cryopreservation can have a tremendous impact upon viability and function.13–15 Nevertheless, some authors have reported equivalent results in functional assays for fresh and frozen samples, when using an optimized protocol.15–18 A precise and rigorous evaluation of the impact of cryopreservation is required to interpret the results of such studies. Assays must be adapted and validated for cryopreserved PBMCs, and the quality of frozen cells has to be monitored to ensure reliable results in functional and phenotypic assays.
To prevent lethal ice crystal formation in frozen cells, 10% dimethyl sulfoxide (DMSO), a polar aprotic solvent, is commonly added to fetal calf serum (FCS) to create the most frequently used freezing solution for cells. But FCS risks transmission of potentially infectious agents to the cell preparation. The use of FCS for cells dedicated for any cell therapy in humans can absolutely be ruled out, because the potential presence of viruses, growth factors, and/or cytokines can be deleterious to patients.19–22
Besides, FCS contains a natural mix of growth factors, hormones, and nutrients supporting cell survival and proliferation but making it inappropriate for studies involving the human immune system. It also contains many uncharacterized components, causing unspecific T-cell activation, so the fluctuating composition prevents standardized cell preparation and analysis. This variability results in batch dependency and can skew immunologic assessment studies done following cell thaw.23 For that reason, there exists the necessity to test every new FCS batch on mitogenic and immune-modulating properties. This FCS pretesting, using control samples from donors with known reactivities to identify batches with low background reactivity and optimal antigen response, is time and cost intensive. In addition, extensive vaccine trials depend on huge amounts of cryomedium, although appropriate FCS batches are limited in their availability. Further, successful vaccine research depends on worldwide sample exchange and comparability of results between collaborating laboratories in multicenter studies. But strict import restrictions on FCS prevent an unlimited availability of frozen samples in laboratories, resulting in difficult conditions for research. Therefore, a number of efforts have been made to develop serum-free growth and cryopreservation media in recent years.24,25
Additionally, DMSO concentration must be reduced significantly because of its toxic impact on cells at room temperature. Even though many publications have already demonstrated the appropriateness of hydroxyethyl starch (HES) to substitute DMSO,26–29 there are no investigations about the usability of HES for the cryopreservation of complete PBMC populations. In addition, the effect of HES on T-cell functionality has not been analyzed until now.
Our intention, in the context of The Global HIV Vaccine Research Cryorepository (GHRC), was the development of standardized methods for collection, cryopreservation, and long-term storage of specimens30 to guarantee the supply of high-quality biological material. The GHRC, established as Central Service Facility for the Collaboration for AIDS vaccine discovery funded by the Bill & Melinda Gates Foundation, is an advanced HIV specimen repository to support the global HIV vaccine research by collecting and offering cryopreserved HIV specimens of relevance for vaccine development to researchers.
Because of this, we have focused on the substitution of the undesirable FCS as cryoprotectant. These substitutes should be safe, chemically defined, available in large quantities, and not import restricted. Besides, the cell viability, cell recovery, and the preservation of immune response have to be ensured.
In this study, we compared different additives to the widely used FCS and identified bovine serum albumin (BSA) fraction V as an appropriate substitute for the potentially immune-modulating FCS. Further, it was possible to reduce the DMSO concentration by the addition of HES as extracellular cryoprotectant. In summary, we developed two serum-free cryopreservation media, produced under GMP conditions, one containing a reduced amount of the cytotoxic and mutagenic DMSO. These media will improve the worldwide sample exchange and the comparability of results created in different laboratories, because of an improved standardization and nonexistent import restrictions. Another benefit is that there are no limitations in the availability of the cryomedia, because they are not subject to batch variations. For monitoring T-cell response, quantitative ELISpot31 was used, because this assay reports the fraction of T-cells that produce a particular cytokine in response to a specific antigen.
Materials and Methods
Sample collection, processing, and handling
Blood samples of 10 healthy, cytomegalus virus (CMV)-seropositive donors were obtained from the blood donor center Saarbruecken with informed consent of the donors. PBMCs were isolated from citrated blood by density gradient centrifugation over lymphocyte separation medium (PAA). The buffy coat layers were collected, pooled, and washed with PBS (Gibco). Contaminating red blood cells were lysed using Pharm Lyse (BD) by incubating 2×108 cells in 20 mL of 1:10 diluted Pharm Lyse in distilled water (B. Braun) for 30 minutes in the dark. Reaction was stopped by adding 30 mL of PBS with 1% FCS.
Media used for cryopreservation
We used 3 different cryomedia to freeze isolated PBMCs. The GHRC-CryoMedium I contains 12.5% BSA fraction V in RPMI 1640 (PAA) supplemented with 10% DMSO. GHRC-CryoMedium II is 12.5% BSA fraction V and 6% HES (MW: 212 kDa) in RPMI 1640 (PAA) with a final DMSO concentration of 5%. FCS as cryomedium (Gemini Bio-Products) was supplemented with DMSO to a final concentration of 10%.
Both GHRC-CryoMedia were prepared in 2 parts. Part A contained no DMSO. For part B, DMSO (Sigma-Aldrich) was added to a concentration of 20% or 10%. To generate the FCS-containing medium, DMSO was added to heat-inactivated FCS to a final concentration of 10%. All cryomedia were freshly prepared and chilled at 4°C.
Cryopreservation of PBMCs
Density gradient–isolated PBMCs were directly resuspended in the FCS-containing cryomedium at a final concentration of 20×106 cells/mL. For cryopreservation using GHRC-CryoMedia I and II, PBMCs were resuspended in GHRC-CryoMedium part A at 40×106 cells/mL. GHRC-CryoMedium part B was added dropwise, resulting in a final concentration of 20×106 cells/mL.
Aliquots of cell suspension (1 mL) were immediately transferred to precooled (−20°C) cryovials (Sarstedt), placed in a frozen thermal pack (−20°C), transferred into a freezing isopropanol container (VWR; cooling rate of 1°C/min) for freezing, and stored at −80°C overnight. Then samples were transferred to the gas phase of a liquid nitrogen storage tank for at least 4 weeks.
Thawing PBMCs
IMDM medium (Gibco) containing l-glutamine, 25 mM HEPES buffer, and 3024 mg/L sodium bicarbonate was supplemented with 10% heat-inactivated FCS (PAA) and 1 L-glutamine (Gibco). Nine milliliters were aliquoted into 50-mL polypropylene tubes (Sarstedt) and warmed to 37°C. Upon removal from liquid nitrogen storage, no more than 2 cryovials were thawed in a 37°C water bath until the cell suspension was melting and a little ice remained. One milliliter of the medium was slowly added to the thawed PBMCs and then transferred to the corresponding polypropylene tube. The tubes were centrifuged at 400 g for 5 min. The PBMCs were resuspended in 10 mL medium and transferred in a cell incubator (5% CO2, 37°C) overnight, with the cap of the tube loosened.
Determination of PBMC viability and recovery
Cell recovery and cell viability were assessed immediately after thawing and after overnight rest using trypan blue exclusion by ViCell (Beckman Coulter). Each sample was measured 3 times.
Cell recovery and cell viability were calculated in the following way:
Recovery (%) (directly after thawing, day 1):
% Recovery=Number of viable PBMCs after thawing×100/Number of frozen viable PBMCs
Recovery (%) (after overnight rest, day 2):
% Recovery=Number of viable PBMCs after overnight rest×100/(Number of frozen viable PBMCs−Number of viable PBMCs removed for measurement on day 1)
% Viability=Number of viable PBMCs×100/Number of total PBMCs
Interferon-γ ELISpot assay
PBMCs were assayed for interferon (IFN)-γ production in the presence of cytomegalo virus (CMV) pp65 peptide pool (BD Bioscience), cytomegalo virus, Epstein-Barr, flu virus (CEF) peptide pool (CTL), PHA (Sigma-Aldrich), and media containing 0.4% DMSO in triplicates. Anti-h-IFN-γ mAb 1-D1k–precoated (Mabtech) 96-well plates were washed 4 times with PBS (Gibco) and blocked with IMDM containing 10% FCS (PAA) and 1 mM l-glutamine for 30 min. Cryopreserved PBMCs were thawed as described earlier and used the next day. Approximately 2×105 PBMCs were added to the CEF, CMV, and background wells and 1×105 PBMCs to the PHA wells. CEF peptides, CMV peptides, and PHA were added to a final concentration of 128, 483, and 8 μg/mL, respectively. The plates were incubated at 37°C under 5% CO2 for 20–22 hours. After washing the plates 5 times with PBS, the production of IFN-γ by T-cells was measured by addition of 1:200 diluted HRP-labeled mAb 7-B6-1 (Mabtech) in sterile and filtered PBS containing 0.5% FCS. After incubation, the plates were washed 5 times with PBS. The spots were developed using Nova Red Substrate Kit (Vector). Spot development was stopped after approximately 1–2 min by extensively washing with distilled water. The spots were evaluated with the Immunopspot Analyzer (CTL). The results were expressed as spot-forming cells (SFCs per million PBMCs).
Statistical analysis
As a Gaussian distribution cannot be assumed using different blood donors, differences in PBMC recovery and viability between the cryomedia were assessed by Wilcoxon signed-rank test, a nonparametric statistical hypothesis test.
Results
PBMC recovery and viability
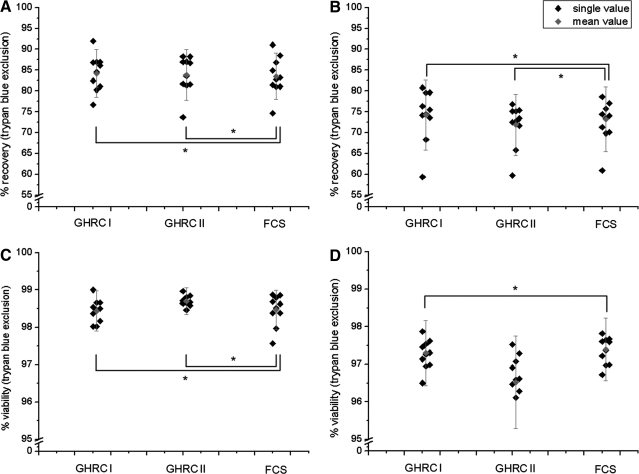
PBMCs from 10 CMV-seropositive healthy donors were cryopreserved in GHRC-CryoMedium I (10% DMSO) and II (5% DMSO) and 90% FCS supplemented with 10% DMSO. From each donor, 6 samples of each cryomedium were thawed and analyzed concerning cell recovery (Fig. 1A, B) and cell viability (Fig. 1C, D) using trypan blue exclusion directly after thawing and after overnight rest.
FIG. 1.
Determination of PBMC recovery (A, B) and viability (C, D) directly after thawing (day 1; A, C) and after overnight rest (day 2; B, D). Single values of 10 independent test series and the mean value with standard deviation after cryopreservation of PBMCs in GHRC-CryoMedium I (10% DMSO) and II (5% DMSO) and 90% FCS supplemented with 10% DMSO were presented. *Statistically equal to FCS (P≤0.05). GHRC, Global HIV Vaccine Research Cryorepository; PBMCs, peripheral blood mononuclear cells; FCS, fetal calf serum; DMSO, dimethyl sulfoxide.
The median recovery immediately after thawing was 84.23% (±5.71%) (GHRC–CryoMedium I), 83.85% (±6.10%) (GHRC-CryoMedium II), and 83.52% (±5.59%) (FCS containing cryomedium) of the initially cryopreserved PBMCs (Fig. 1A).
The recovery immediately after thawing was greater than observed after overnight culture of the PBMCs. Thus, we detected a mean PBMC recovery of 74.27% (±8.42%), 71.85% (±7.36%), and 73.21% (±7.75%) using GHRC-CryoMedia I and II and FCS-containing cryomedium, respectively (Fig. 1B).
Statistical analysis using the Wilcoxon signed-rank test showed that cryopreservation with both GHRC-CryoMedia resulted in equal recovery values directly after thawing and after overnight rest compared to FCS containing 10% DMSO.
The mean PBMC viability was greater than 98% after thawing (Fig. 1C) and 96% after overnight rest (Fig. 1D) for all 3 cryomedia used.
The median viability immediately after thawing was 98.44% (±0.54%), 98.70% (±0.36%), and 98.47% (±0.53%) of the initially cryopreserved PBMCs using GHRC-CryoMedia I and II and FCS-containing cryomedium, respectively.
The viability immediately after thawing was greater than that observed after overnight culture of the PBMCs. So, we detected a mean PBMC viability of 97.30% (±0.87%) (GHRC I), 96.52% (±1.24%) (GHRC II), and 97.39% (±0.84%) (FCS).
Statistical analysis using the Wilcoxon signed-rank test showed that cryopreservation using GHRC I resulted in equal viability of PBMCs when compared to FCS containing 10% DMSO, whereas with GHRC II, viability was reduced after overnight rest of the cells.
Characterization of T-cell response in IFN-γ ELISpot assay
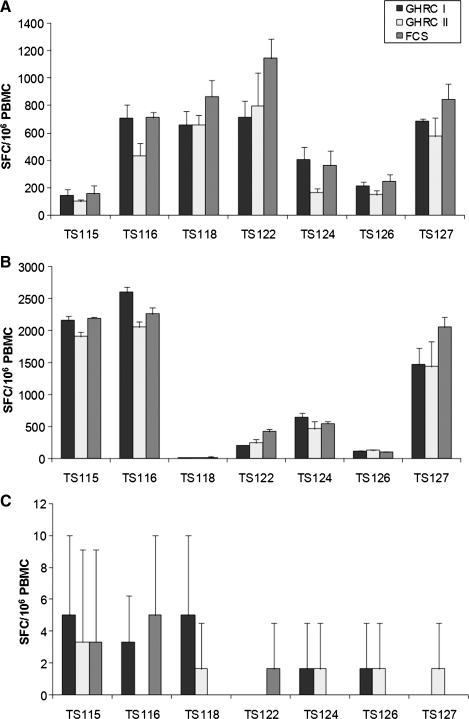
We could demonstrate that our 2 new cryomedia resulted in nearly similar cell recovery and cell viability compared to the commonly used FCS as cryoprotectant. Our aim was also to determine whether functional T-cell response after cryopreservation in our 2 cryomedia was comparable to the results using FCS-based media. Therefore, PBMCs were tested in the IFN-γ ELISpot using CEF (Fig. 2A) and CMV (Fig. 2B) peptide pools as immunogenic antigens and enumerated in replicates of 3 wells.
FIG. 2.
Enzyme-linked immunospot response of 7 donors (TS) stimulated with cytomegalo virus Epstein-Barr virus, flu virus (CEF) (A) and cytomegalo virus (CMV) peptide pool (B) as well as the background signal of unstimulated PBMCs (C) after cryopreservation in GHRC-CryoMedia I (10% DMSO) and II (5% DMSO) and 90% FCS supplemented with 10% DMSO. Values shown are SFCs/106 PBMCs from 3 replicate wells. Missing bars indicate no unspecific response. SFCs, spot-forming cells.
To compute a cutoff for positive responses, the average number of SFCs/106 PBMCs was determined; 3 replicates were used for this calculation. Following “Standardization and Validation Issues of ELISpot Assay,”32 we used the definition of responder R>4D and R>50 [R=reagent (CMV peptide pool, CEF peptide pool)] SFCs/106 PBMCs, where D corresponds to SFCs for diluent (background).
Using the definition, all 7 and 6 of 7 PBMC samples had a response to the CEF (Fig. 2A) and CMV (Fig. 2B) peptide pool for all 3 cryomedia, respectively. In addition, the background was also very low with all cryomedia used (Fig. 2C).
To compare the specific reactivity of the frozen PBMCs between the 3 different cryomedia, we classified the INF-γ ELISpot reactivity in 4 different groups: negative <50 SFCs/106 PBMCs, low 51–250 SFCs/106 PBMCs, medium 251–1000 SFCs/106 PBMCs, and high >1000 SFCs/106 PBMCs (Tables 1 and 2).
Table 1.
Reactivity of PBMCs from 7 Donors Measured by IFN-γ ELISpot Using CEF Peptide Pool as Stimulant After Cryopreservation in GHRC-CryoMedia I (10% DMSO) and II (5% DMSO) and 90% FCS Supplemented with 10% DMSO
| CEF peptide pool | GHRC I | GHRC II | FCS |
|---|---|---|---|
| TS115 | Low | Low | Low |
| TS116 | Medium | Medium | Medium |
| TS118 | Medium | Medium | Medium |
| TS122 | Medium | Medium | Medium |
| TS124 | Medium | Low | Medium |
| TS126 | Low | Low | Low |
| TS127 | Medium | Medium | Medium |
ELISpot, enzyme-linked immunospot; PBMCs, peripheral blood mononuclear cells; GHRC, Global HIV Vaccine Research Cryorepository; FCS, fetal calf serum; DMSO, dimethyl sulfoxide; IFN, interferon; CEF, cytomegalo virus Epstein-Barr virus, flu virus.
Table 2.
Reactivity of PBMCs from 7 Donors Measured by IFN-γ ELISpot Using CMV Peptide Pool as Stimulant After Cryopreservation in GHRC-CryoMedia I (10% DMSO) and II (5% DMSO) and 90% FCS Supplemented with 10% DMSO
| CMV peptide pool | GHRC I | GHRC II | FCS |
|---|---|---|---|
| TS115 | High | High | High |
| TS116 | High | High | High |
| TS118 | Negative | Negative | Negative |
| TS122 | Low | Low | Medium |
| TS124 | Medium | Medium | Medium |
| TS126 | Low | Low | Low |
| TS127 | High | High | High |
CMV, cytomegalo virus.
We could demonstrate that cryopreservation of PBMCs in our new cryomedia GHRC I and II resulted in nearly identical reactivity in IFN-γ ELISpot using this classification. Only 2 outliers were detectable in the media comparison. Using CEF peptide pool as immunogenic antigen, the PBMCs of donor TS124 frozen in GHRC CryoMedium II showed low reactivity in comparison to the other cryomedia. Similarly, the PBMCs of donor TS122 frozen in FCS-containing cryomedia had a higher reactivity with CMV peptide pool as stimulant. So, PBMC cryopreservation using our cryomedia resulted not only in high cell recovery and viability values, but also in a T-cell functionality that was comparable to PBMCs frozen in FCS-based cryomedia.
Discussion
Cryopreservation of cells offers many advantages to the research community, such as banking of multiple aliquots of cells from multicenter studies of large cohorts of individuals. It allows precious samples to be available for future studies, often using newly developed techniques or assays. Additionally, samples of the same donor banked over time can be simultaneously processed, allowing greater inter- and intralaboratory control and reducing costs. But efficient cryopreservation of specimens is crucial for the success of these studies. It was previously shown that the viability of cryopreserved PBMCs has tremendous effects on the results of functional assays,13 so that at least 70% viability is necessary for conclusive responses to antigens and mitogens. PBMCs with viability of 70% can also be used for immunomagnetic cell separation, cytokine production studies, and flow cytometric analyses.33,34 Although cell recovery does not interfere with the results of immunologic assays, better recovery of only one proportion of cells in a heterogeneous population may preclude assay performance altogether. Based on these results, cryopreservation quality assurance programs are necessary for guaranteeing the quality of cryopreserved PBMCs and for increasing standardization between laboratories. For an efficient cryopreservation, FCS is used in most freezing media although each serum batch is unique in its ability to support optimal assay resolution and may potentially contain mitogenic or immune suppressive factors. It was already reported by The Cancer Vaccine Consortium of the Sabin Vaccine Institute that apparently the serum choice among their participants was responsible for suboptimal performance in one of their international ELISpot proficiency panels.35 However, to avoid import restrictions and negative impacts of viruses, growth factors and cytokines, human serum can be used for cryopreservation and immunologic assessment studies. But the cost of human serum is high and makes immunotherapy clinical trials, which are performed in large scale since the 1980s, unaffordable.
Because PBMC cryopreservation should be optimized and standardized to avoid affecting T-cell assays, we investigated the efficiency of novel serum-free and DMSO-reduced cryopreservation media by analyzing cell viability and recovery, as well as maintenance of T-cell functionality. Although some publications have recently been published describing the benefit of serum-free media in ELISpot assays with PBMCs cryopreserved in serum-containing media36,37 the culture medium for T-cells is in general still supplemented with serum.38–42 Additionally, we could detect significant differences in the outcome of ELISpot assays after PBMC cryopreservation in various FCS-based cryomedia, using pretested serum as medium supplement for ELISpot analysis (data not shown). As cryopreservation itself seems to influence the T-cell functionality considerably, independent of the medium used for PBMC cultivation and analysis, we focused our investigation on the replacement of FCS-containing cryomedia by serum-free compositions.
In this process, we could demonstrate that BSA fraction V is an appropriate candidate as a cryoprotectant to substitute the potentially immune-modulating FCS. Besides, with the addition of HES the reduction of the toxic DMSO concentration was possible, resulting only in a very slight decrease of the PBMC recovery, because the use of HES caused an increase of the glass transition temperature and the viscosity of the freezing medium.43 Extracellular cryoprotective agents, such as HES, usually withdraw the water and protect the cells from osmotically and electrically induced membrane damages from outside. In contrast, intracellular DMSO replaces the water shell in macromolecules, so that cells are able to survive intracellular ice crystallization.44,45 Moreover, adverse impacts on the function of antigen-specific lymphocytes at thawing were determined using IFN-γ ELISpot assay. At this, the focus was not on comparing the T-cell functionality between fresh isolated and cryopreserved PBMCs, but on demonstrating that T-cell response after cryopreservation in the new cryomedia was similar to FCS.
Our results were facilitated by other investigations that demonstrated that the function of cryopreserved PBMCs was associated with viability46 and that the type of protein additive in the cryopreservation media is critical for viability and antigen-specific function of T-cells.47 A multitude of studies already presented the disadvantageous effects of cryopreservation on the function of lymphocytes in antigen-specific T-cell assays. Cryopreservation of lymphocytes may have effects on cell surface molecules of T-cells such as CCR5 and CD45 RA/RO and may decrease responses to infectious diseases and recall antigens10 in both HIV-infected and noninfected volunteer donor blood. Further, cryopreservation can modify the ability of T-cells to secrete cytokines. Freezing and thawing cells hardly affected the cytokine secretion of cells from volunteer donors,15 and other investigators have demonstrated considerable differences after cryopreservation regarding the antigen-specific cytokine secretion of T-cells from cancer patients.18 The reliable evaluation of cryopreserved T-cell responses at time points distant from clinical trials is necessary for studies of immune-based therapies. The technology for sample analysis makes further progress, and adequate equipment and technical proficiency is not always available on site. Finally, an expanding number of patients across multiple sites will be necessary to conduct clinical trials, and consequently, analyzing T-cell responses on freshly isolated PBMCs will be impracticable.
On the basis of data described here, we developed 2 different serum-free cryopreservation media, one of them with a reduced amount of DMSO, produced under GMP conditions. Both cryomedia resulted in very good viability, recovery, and functionality of PBMCs after cryopreservation, compared to a commonly used FCS-containing cryomedium. The DMSO reduction by addition of HES revealed only marginal differences in the experimental outcome, indicating a promising approach for further improvements. Using these cryomedia, frozen samples can be transported without import restrictions, an important facilitation for clinical trials and in field studies. Besides, it is widely known that the use of serum in antigen-specific T-cell assays may lead to an increased unspecific activation of lymphocytes because of different cytokines and batch-dependent variability in results. Because of that, the serum-free and standardized cryomedia prevent time and cost intensive comparison of the efficiency of different FCS lots and increase reproducibility of results.
In future studies, the complete avoidance of animal proteins and products in combination with efficient cryopreservation SOPs should be the major aim. By minimizing the variety of components, reliability and standardization of results will be increased.
Acknowledgments
The authors thank A. Reich for her excellent technical assistance. This work was financed by a grant from the Bill & Melinda Gates Foundation (grant No. 38580).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Shearer WT. Rosenblatt HM. Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112:973–980. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Zinkernagel RM. Hengartner H. On immunity against infections and vaccines: credo 2004. Scand J Immunol. 2004;60:9–13. doi: 10.1111/j.0300-9475.2004.01460.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- 4.Moorthy VS. Good MF. Hill AV. Malaria vaccine developments. Lancet. 2004;363:150–156. doi: 10.1016/S0140-6736(03)15267-1. [DOI] [PubMed] [Google Scholar]
- 5.Borrow P. Lewicki H. Hahn BH, et al. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altfeld M. Rosenberg ES. Shankarappa R, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosillo MC. Ortuno F. Rivera J, et al. Cryopreservation modifies flow-cytometric analysis of hemopoietic cells. Vox Sang. 1995;68:210. doi: 10.1111/j.1423-0410.1995.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 8.Oldham RK. Dean JH. Cannon GB, et al. Cryopreservation of human lymphocyte function as measured by in vitro assays. Int J Cancer. 1976;18:145–155. doi: 10.1002/ijc.2910180203. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg A. Wohl DA. Brown DG, et al. Effect of cryopreservation on measurement of cytomegalovirus-specific cellular immune responses in HIV infected patients. J Acquir Immune Defic Syndr. 2000;25:109–114. doi: 10.1097/00042560-200010010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Costantini A. Mancini S. Giuliodoro S, et al. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods. 2003;278:145–155. doi: 10.1016/s0022-1759(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 11.Weinberg A. Betensky RA. Zhang L, et al. Effect of shipment, storage, anticoagulant, cell separation on lymphocyte proliferation assays for human immunodeficiency virusinfected patients. Clin Diagn Lab Immunol. 1998;5:804–807. doi: 10.1128/cdli.5.6.804-807.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maecker HT. Dunn HS. Suni MA, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg A. Zhang L. Brown D, et al. Viability and functional activity of cryopreserved mononuclear cells. Clin Diagn Lab Immunol. 2000;7:714–716. doi: 10.1128/cdli.7.4.714-716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleeberger CA. Lyles RH. Margolick JB, et al. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin Diagn Lab Immunol. 1999;6:14–19. doi: 10.1128/cdli.6.1.14-19.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreher CR. Dittrich MT. Guerkov R, et al. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods. 2003;278:79–93. doi: 10.1016/s0022-1759(03)00226-6. [DOI] [PubMed] [Google Scholar]
- 16.Smith JG. Liu X. Kaufhold RM, et al. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicellazoster virus. Clin Diagn Lab Immunol. 2001;8:871–879. doi: 10.1128/CDLI.8.5.871-879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebauer BS. Hricik DE. Atallah A, et al. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant. 2002;2:857–866. doi: 10.1034/j.1600-6143.2002.20908.x. [DOI] [PubMed] [Google Scholar]
- 18.Sobota V. Bubenik J. Indrova M, et al. Use of cryopreserved lymphocytes for assessment of the immunological effects of interferon therapy in renal cell carcinoma patients. J Immunol Methods. 1997;203:1–10. doi: 10.1016/s0022-1759(97)00020-3. [DOI] [PubMed] [Google Scholar]
- 19.Nuttall PA. Luther PD. Stott EJ. Viral contamination of bovine foetal serum and cell cultures. Nature. 1977;266:835–837. doi: 10.1038/266835a0. [DOI] [PubMed] [Google Scholar]
- 20.Erickson GA. Bolin SR. Landgraf JG. Viral contamination of fetal bovine serum used for tissue culture: risks and concerns. Dev Biol Stand. 1991;75:173–175. [PubMed] [Google Scholar]
- 21.Weber DJ. Biosafety considerations for cell-based therapies. BioPharm Int. 2004;17:48–55. [Google Scholar]
- 22.Zabal O. Kobrak AL. Lager IA, et al. Contamination of bovine fetal serum with bovine viral diarrhea virus. Rev Argent Microbiol. 2000;32:27–32. [PubMed] [Google Scholar]
- 23.Best A. Hidalgo G. Mitchell K, et al. Issues concerning the large scale cryopreservation of peripheral blood mononuclear cells (PBMC) for immunotherapytrials. Cryobiology. 2007;54:294–297. doi: 10.1016/j.cryobiol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Froud SJ. The development, benefits and disadvantages of serumfree media. Dev Biol Stand. 1999;99:157–166. [PubMed] [Google Scholar]
- 25.Campbell LH. Brockbank KG. Serum-free solutions for cryopreservation of cells. In Vitro Cell Dev Biol Anim. 2007;43:269–275. doi: 10.1007/s11626-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 26.Kenmochi T. Asano T. Maruyama M, et al. Cryopreservation of human pancreatic islets from non-heart-beating donors using hydroxyethyl starch and dimethyl sulfoxide as cryoprotectants. Cell Transplant. 2008;17:61–67. doi: 10.3727/000000008783907026. [DOI] [PubMed] [Google Scholar]
- 27.Clapisson G. Salinas C. Malacher P, et al. Cryopreservaion with hydroxyethylstarch (HES)+dimethylsulfoxide (DMSO) gives better results than DMSO alone. Bull Cancer. 2004;91:E97–E102. [PubMed] [Google Scholar]
- 28.Thomas MJ. Parry ES. Nash SG, et al. A method for the cryopreservation of red blood cells using hydroxyethyl starch as a cryoprotectant. Transfus Sci. 1996;17:385–396. doi: 10.1016/0955-3886(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 29.Stiff PJ. Murgo AJ. Zaroulis CG, et al. Unfractionated human marrow cell cryopreservation using demethylsulfoxide and hydroxyethyl starch. Cryobiology. 1983;20:17–24. doi: 10.1016/0011-2240(83)90054-8. [DOI] [PubMed] [Google Scholar]
- 30.Shreffler WG. Visness CM. Burger M, et al. Standardization and performance evaluation of mononuclear cell cytokine secretion assays in a multicenter study. BMC Immunol. 2006;7:29. doi: 10.1186/1471-2172-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czerkinsky C. Andersson G. Ekre HP, et al. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferonsecreting cells. J Immunol Methods. 1988;110:29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- 32.Thomson S. Methods in molecular biology. In: Kalyuzhny AE, editor. Methods in Molecular Biology, vol. 302, chapter 4. Vol. 302. Human Press; Totowa, NJ: 2005. pp. 75–76. [Google Scholar]
- 33.Sleasman JW. Leon BH. Aleixo LF, et al. Immunomagnetic selection of purified monocyte and lymphocyte populations from peripheral blood mononuclear cells following cryopreservation. Clin Diagn Lab Immunol. 1997;4:653–658. doi: 10.1128/cdli.4.6.653-658.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimann KA. Chernoff M. Wilkening CL, et al. Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. Clin Diagn Lab Immunol. 2000;7:352–359. doi: 10.1128/cdli.7.3.352-359.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janetzki S. Panageas KS. Ben-Porat L, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janetzki S. Price L. Britten CM, et al. Performance of serum-supplemented and serum-free media in IFNγ Elispot Assays for human T cells. Cancer Immunol Immunother. 2010;59:609–618. doi: 10.1007/s00262-009-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mander A. Gouttefangeas C. Ottensmeier C, et al. Serum is not required for ex vivo IFN-γ ELISPOT: a collaborative study of different protocols from the European CIMT Immunoguiding Program. Cancer Immunol Immunother. 2010;59:619–627. doi: 10.1007/s00262-009-0814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper LJ. Ausubel L. Gutierrez M, et al. Manufacturing of gene-modified cytotoxic T lymphocytes for autologous cellular therapy for lymphoma. Cytotherapy. 2006;8:105–117. doi: 10.1080/14653240600620176. [DOI] [PubMed] [Google Scholar]
- 39.Foster AE. Forrester K. Gottlieb DJ, et al. Large-scale expansion of cytomegalovirus-specific cytotoxic T cells in suspension culture. Biotechnol Bioeng. 2004;85:138–146. doi: 10.1002/bit.10801. [DOI] [PubMed] [Google Scholar]
- 40.Yssel H. De Vries JE. Koken M, et al. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. J Immunol Methods. 1984;72:219–227. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 41.Baba T. Sato-Matsushita M. Kanamoto A, et al. Phase I clinical trial of the vaccination for the patients with metastatic melanoma using gp100-derived epitope peptide restricted to HLA-A*2402. J Transl Med. 2010;8:84. doi: 10.1186/1479-5876-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slingluff CL., Jr. Petroni GR. Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 43.Levine H. Slade L. Water as a plasticizer: physico-chemical aspects of low moisture polymeric systems. In: Franks F, editor. Water Science Reviews, Vol. III. Cambridge Univ. Press; 1987. pp. 79–185. [Google Scholar]
- 44.Lovelock JE. The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochim Biophys Acta. 1953;11:28–36. doi: 10.1016/0006-3002(53)90005-5. [DOI] [PubMed] [Google Scholar]
- 45.Murthy SSN. Some insight into the physical basis of the cryoprotective action of dimethyl sulfoxide and ethylene glycol. Cryobiology. 1998;36:85–96. doi: 10.1006/cryo.1997.2064. [DOI] [PubMed] [Google Scholar]
- 46.Betensky RA. Connick E. Devers J, et al. Shipment impairs lymphocyte proliferative responses to microbial antigens. Clin Diagn Lab Immunol. 2000;7:759. doi: 10.1128/cdli.7.5.759-763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Disis ML. dela Rosa C. Goodell V, et al. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J Immunol Methods. 2006;308:13–18. doi: 10.1016/j.jim.2005.09.011. [DOI] [PubMed] [Google Scholar]