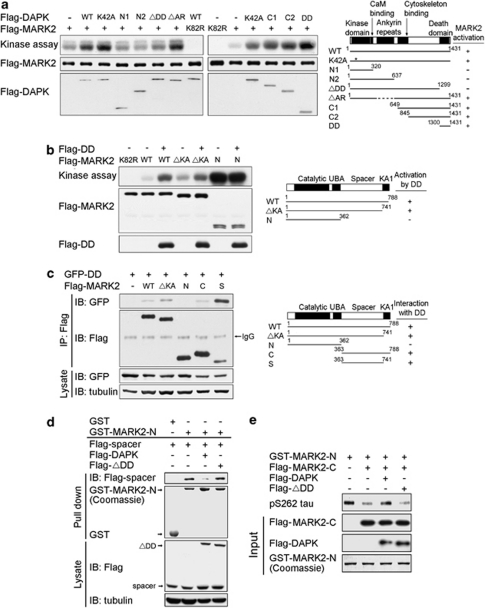
Figure 4.
DAPK relieves MARK2 autoinhibition by binding to MARK2 spacer region. (a) DAPK DD is responsible for MARK2 activation. Various DAPK fragments and full-length MARK2 purified from baculovirus were incubated in the kinase reaction with tau-C as the substrate. Substrate phosphorylation was detected by autoradiography (upper panel) and the input amounts of MARK2 and DAPK fragments were detected by immunoblot (lower panels). Schematics of DAPK mutants and their ability to activate MARK2 are shown on the right. (b) The spacer region is required for MARK2 activation by DAPK. Baculovirally purified DAPK DD (DD) was incubated with baculovirally purified MARK2 fragments and assayed for MARK2-mediated tau-C phosphorylation as in a. Schematics of MARK2 mutants and their responsiveness to DAPK DD are shown on the right. (c) DAPK DD interacts with MARK2 spacer region. 293T cells were co-transfected with GFP-DD (DAPK DD fused with GFP) and various Flag-MARK2 fragments as indicated. Cell lysates were used for immunoprecipitation with anti-Flag antibody. The immunoprecipitates and cell lysates were analyzed by immunoblot with antibodies as indicated. Schematics of MARK2 mutants and their ability to bind DAPK DD are shown on the right. (d) DAPK DD disrupts MARK2 intramolecular interaction. 293T cells transfected with Flag-tagged DAPK, DAPKΔDD (ΔDD), and/or MARK2 spacer region (spacer) were lysed and cell lysates were incubated with bacterially purified GST or GST-MARK2-N. Bound proteins were analyzed by immunoblot with anti-Flag antibody. The input amounts of GST fusion proteins and the expression of Flag-tagged proteins are shown on the lower panels. (e) DAPK relieves MARK2 autoinhibition. GST-MARK2-N purified from bacteria and various Flag-tagged proteins purified from baculovirus were incubated in the kinase reaction as in a. Tau phosphorylation was detected by immunoblot with pS262 tau antibody and the equal inputs of various proteins are shown on the lower panels