Abstract
A bidirectional association between mood disorders and cardiovascular disease has been described; however, the neurobiological mechanisms that underlie this link have not been fully elucidated. The purpose of this review is first to describe some of the important behavioral neurobiological processes that are common to both mood and cardiovascular disorders. Second, this review focuses on the value of conducting research with animal models (primarily rodents) to investigate potential behavioral, physiological, and neural processes involved in the association of mood disorders and cardiovascular disease. In combination with findings from human research, the study of mechanisms underlying mood and cardiovascular regulation using animal models will enhance our understanding of the association of depression and cardiovascular disease, and can promote the development of novel interventions for individuals with these comorbid conditions.
Keywords: Autonomic nervous system, Behavior, Chronic mild stress, Cytokines, Depression, Endocrine system, Heart disease, Hypothalamic-pituitary-adrenal axis, Immune system, Oxytocin, Prairie vole, Rodent models, Social isolation
Emotion and the Cardiovascular System: An Important Public Health Concern
The purpose of this article is to discuss the association between emotion and cardiovascular regulation, focusing on the value of utilizing animal models to investigate neurobiological mechanisms. A specific example of this association is the link between depression and cardiovascular disease (CVD). This link is bidirectional, such that dysfunction of the cardiovascular system has a significant influence on mood; and negative mood has a significant influence on cardiac morbidity and mortality (Barefoot & Schroll, 1996; Carney & Freedland, 2003; Frasure-Smith et al., 1995; Freedland et al., 2003; Glassman, 2007; Penninx et al., 2001; Van der Kooy et al., 2007). This link is an important public health concern. For example, CVD and depression are two of the most detrimental health conditions in developed countries (Mathers & Loncar, 2005). The association of these conditions is observed in individuals both with and without a history of cardiac problems, and is independent of traditional cardiovascular risk factors such as hypertension, high cholesterol, and family history (Carney & Freedland, 2003; Frasure-Smith & Lésperance, 2003; Penninx et al., 2001; Wulsin & Singal, 2003).
Many theories have been postulated to explain the association between mood and CVD (de Jonge et al., 2010; Evans & Charney, 2004; Freedland et al., 2006; Glassman, 2007; Steptoe & Whitehead, 2005), however our current understanding of this link is limited. Several barriers prevent the development of comprehensive theories to describe the mechanisms of interaction between emotion and cardiovascular regulation. Challenges to our understanding originate in part from the lack of useful “biological markers” of emotion-related disorders (see Mössner et al., 2007), coupled with the limited number of experimental investigations of biopsychosocial processes underlying these conditions. Therefore, in combination with studies involving human samples, integrative research that employs valid and reliable animal models will improve our understanding of mechanisms that influence the association of emotion and cardiovascular function.
The Value of Animal Models for the Study of Emotion and Cardiovascular Function
Research that utilizes animal model systems offers several novel advantages for the study of mood and cardiovascular function. This approach allows for a high level of experimental control, integrative methods and analyses, and the potential for studying causal neurobiological and behavioral mechanisms. A focus on animal disease models that have translational relevance to humans, along with using validated methodological procedures, will allow for studies of common and causal mechanisms involved in regulating emotion and the cardiovascular system. Strategies that emphasize core behavioral and neurobiological features of emotion-regulation disorders and CVDs will contribute significant insights, especially when these features are carefully defined, observed, quantified, and systematically investigated.
Several animal models of mood disorders have been developed and validated (Anisman & Matheson, 2005; Cryan & Slattery, 2007; Frazer & Morilak, 2005; Matthews et al., 2005; Willner, 2005). These models have proven to be very useful for the study of neurobiological and behavioral correlates of mood disorders. For instance, non-human primate models involving cynomolgus monkeys have focused on vascular responsiveness, neurotransmitter functions, and social behaviors in the context of depression and/or CVD (Hamm Jr. et al., 1983; Shively & Bethea, 2004). Rodent models such as learned helplessness (Maier & Watkins, 2005; Seligman, 1974), exposure to chronic unpredictable stressors (Katz, 1982; Willner, 2005), and behavioral despair (Cryan et al., 2005; Porsolt et al., 1977) have focused on behavioral and physiological consequences of depression, mechanisms of antidepressant treatments, and potential etiological factors in mood disorders. Newer models in rodents have investigated the role of the social environment in mediating affective signs and autonomic function (Bartolomucci et al., 2003; Grippo et al., 2007b; Sgoifo et al., 2001).
To highlight the value of animal models, the following sections will explore the substantive contributions from animal research regarding several mechanisms that may underlie negative mood and CVD. The following mechanisms will be addressed here, including a focus on evidence derived from both human and non-human animal studies: (1) interactions of stress and the autonomic nervous system; (2) social stress and the disruption of social bonds; (3) interactions of the endocrine and immune systems; and (4) dysfunction of central nervous system processes. In addition, the sections below will highlight three specific rodent models that have contributed to our understanding of behavioral, physiological, and neural mediators of mood and cardiovascular function, including: (1) the chronic mild stress model of depression; (2) a model of experimental heart disease (heart failure); and (3) a model of disrupted social bonds.
Stress and the Autonomic Nervous System
Interactions of Environmental Stressors and Autonomic Regulation of the Heart
An exaggerated response to stressors influences the development and maintenance of mood disorders and CVDs. The presence of chronic, unpredictable, or uncontrollable stressors may play an important role in the development of depressive signs and symptoms (Anisman & Matheson, 2005) and cardiovascular dysregulation (Sgoifo et al., 2001), as it is difficult for an organism to adapt to these stressors. Exposure to uncontrollable stressors contributes to CVD and its antecedent risk factors, such as hypertension, changes in vascular resistance, endothelial dysfunction, and ventricular arrhythmias, among others (Bairey Merz et al., 2002; Johnson & Anderson, 1990; Sanders & Lawler, 1992; Schwartz et al., 2003). The predisposing influence of environmental stressors on depression has been reviewed in detail elsewhere; several lines of evidence indicate that uncontrollable and unpredictable stressors are associated with depressive syndromes in humans and depression-like behaviors in animal models (Anisman & Matheson, 2005; Monroe & Harkness, 2005; Swaab et al., 2005).
A physiological mechanism by which stress interacts with both mood and cardiovascular function is through the autonomic nervous system. Depressive disorders are characterized by changes in autonomic function, including activation of the sympathetic nervous system, withdrawal of parasympathetic regulation the heart, elevated heart rate, and reduced heart rate variability (i.e., rhythmic changes in heart rate to adjust to beat-by-beat perturbations to the cardiovascular system) (Barton et al., 2007; Carney et al., 1995; Krittayaphong et al., 1997; Pitzalis et al., 2001; Watkins & Grossman, 1999). These autonomic changes are associated with cardiovascular risk factors such as hypertension, increased body mass index, and increased blood glucose, and have been observed in both acute and chronic cardiovascular conditions (Carney et al., 1993; Esler & Kaye, 2000; Kannel et al., 1987; Kristal-Boneh et al., 1995; La Rovere et al., 1998). Therefore, depression may influence directly the development and/or progression of CVD via effects on autonomic imbalance, cardiac rate or rhythm disturbances, or electrical instability. Through feedback to the brain, these changes may influence endocrine and immune function, as well as behavior, leading to additional changes at the level of the cardiovascular system to perpetuate the disease process.
The Utility of Animal Models for Investigating Stress and the Autonomic Nervous System
The precise autonomic and cardiac changes associated with depressive disorders are not well characterized and have yielded some inconsistent results (Barton et al., 2007; Watkins et al., 2002). Therefore, examining cardiovascular and other physiological consequences in the chronic mild stress (CMS) rodent model of depression is useful for gaining insight into mechanisms involved in cardiovascular dysregulation associated with depression. The CMS model involves exposing rodents to a chronic period of unpredictable, mild stressors, such as strobe light, ambient noise, or damp bedding. These mild – yet unpredictable and uncontrollable – stressors are considered to realistically mimic daily hassles that humans experience. Initial studies with this model have been useful for examining behavioral changes in depression as well as the efficacy of antidepressant treatments (Katz, 1982; Willner et al., 1987; Willner et al., 1994). The validity and reliability of CMS as a model of depression have been described in detail previously (Willner, 1997; Willner, 2005).
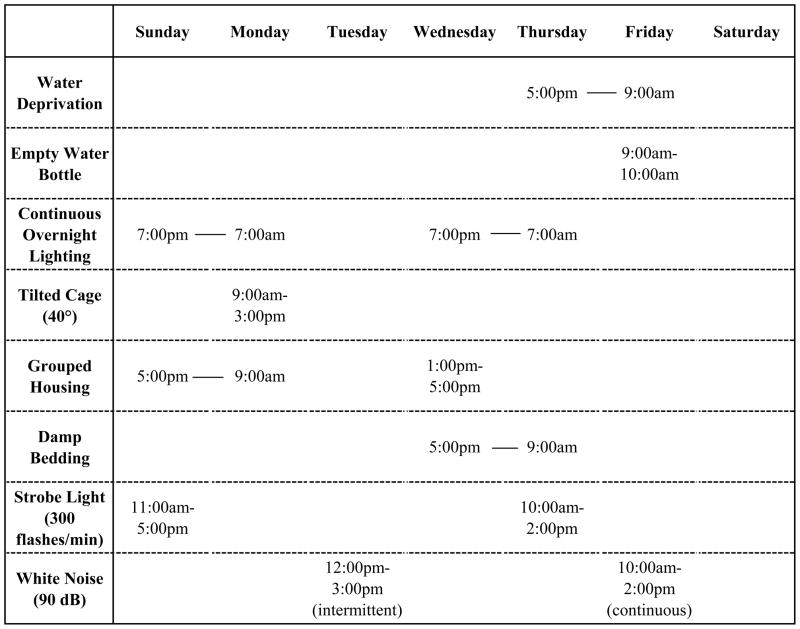
In a series of studies focusing specifically on autonomic and cardiovascular consequences associated with depression, male rats were exposed to 4 weeks of CMS, which included the following brief stressors (see Figure 1 for an example CMS paradigm): continuous overnight lighting; paired housing; a tilted cage; damp bedding; white noise; strobe light; and overnight water deprivation followed by exposure to an empty water bottle (Grippo et al., 2002; Grippo et al., 2003a; Grippo et al., 2006). The combination of these stressors produces behavioral changes consistent with depressive syndromes, including anhedonia, which is the reduced responsiveness to pleasurable experiences (reduced intake of a sucrose solution), and reduced physical activity (reduced spontaneous activity in a running wheel). Coupled with these behavioral changes, CMS produces several cardiovascular alterations consistent with CVD, including elevated resting heart rate, reduced heart rate variability, and exaggerated responsiveness to stressors (Grippo et al., 2002; Grippo et al., 2003a).
Figure 1.
A sample chronic mild stress (CMS) paradigm used in studies to investigate the association between depressive behaviors and autonomic dysfunction.
While the behavioral changes associated with CMS recover within a few weeks following cessation of the stressors, the cardiovascular disruptions do not recover at the same rate. For example, at a time point when anhedonia and reduced activity are no longer observable after cessation of the CMS paradigm, animals continue to display an increase in resting heart rate, a decrease in heart rate variability, and exaggerated cardiac reactivity to stress (Grippo et al., 2003a). These findings suggest that simple remediation of the depressive signs is not associated with alleviation of the underlying cardiovascular pathophysiology. Consistent with these findings are those from Carney et al. (2000), suggesting that pharmacotherapy or psychotherapy for depression may partially improve heart rate and heart rate variability, but may not be sufficient to repair cardiovascular status to baseline levels.
Given these previous findings, it is important to gain a greater comprehension of the specific mechanisms of cardiovascular changes associated with negative mood, to aid in the development of more effective treatments for patients with depression and CVD. Studies with the CMS model have implicated the sympathetic nervous system as an important mechanism. Selective blockade of sympathetic inputs to the heart with a drug (propranolol hydrochloride) indicates that sympathetic drive is elevated in rats exposed to CMS, similar to the excess sympathetic drive observed in patients with CVD (Grippo et al., 2002; Grippo et al., 2003a). In addition, CMS is associated with an increased vulnerability to ventricular arrhythmias when the cardiovascular system is challenged with a pro-arrhythmic drug (aconitine) (Grippo et al., 2004). Depression may therefore be associated with exaggerated sympathetic drive to the heart and ventricular electrical instability, which in turn influences cardiovascular function and disease outcomes. These results are especially significant when considered in the context of findings from human populations. For example, patients with both CVD and depression have a higher prevalence of ventricular arrhythmias, compared with patients that are not experiencing depression (Carney et al., 1993). Similarly, following myocardial infarction (e.g., “heart attack”) patients are at a greater risk of mortality if they have a combination of ventricular arrhythmias and high depressive symptomatology, relative to patients with fewer arrhythmias or those with a lower depression score (Frasure-Smith et al., 1995).
Social Stress and the Disruption of Social Bonds
Mechanisms Linking Social Stress, Depression, and Heart Disease
The association of mood and cardiovascular disorders may be modulated by specific reactions to social stressors, including social isolation or the lack of positive social interactions. The social environment clearly is an important factor in the daily lives of humans and other social mammals. Research from human participants suggests that the disruption of social bonds and perceived loneliness are associated with maladaptive grief, mood disorders, and autonomic imbalance, as well as dysfunctional interactions among these variables (Berkman et al., 2004; Cacioppo et al., 2002; Cacioppo & Hawkley, 2003; Steptoe et al., 2004; Thurston & Kubzansky, 2009). Individuals with smaller social networks (e.g., being unmarried, having fewer close friends, or having fewer associations with community organizations) have shown increased depressive symptomatology (Rutledge et al., 2008). Further, social isolation and fewer social connections also are associated with several cardiovascular risk factors, including coronary artery calcification, increased blood glucose levels, hypertension, and diabetes; and are linked to an increased risk of cardiovascular mortality in both men and women (Eng et al., 2002; Kaplan et al., 1988; Kop et al., 2005; Ramsay et al., 2008; Rutledge et al., 2004; Rutledge et al., 2008).
The Utility of Animal Models for Investigating Social Stress and the Disruption of Social Bonds
Experimental investigations with animal models indicate that alterations in the social environment produce several negative behavioral and physiological changes consistent with depression and CVD (Bosch et al., 2009; Grippo et al., 2007b; Shively et al., 2002; Shively et al., 2009). For instance, Shively and colleagues have observed behavioral signs of depression, increased heart rate, and atherosclerotic plaques in female cynomolgus monkeys when they are the subordinate members of a social hierarchy (Shively et al., 2002; Shively et al., 2009).
In addition to findings from primates, altered behaviors and autonomic dysfunction have been observed in rodent studies involving social isolation (Grippo et al., 2007b). To investigate the mechanisms by which negative social experiences influence mood and cardiovascular regulation, studies have focused on a specific social rodent species, the prairie vole. Similar to primates, prairie voles are highly dependent on social interactions for the regulation of behavior, endocrine, and autonomic function. These animals are considered socially monogamous and cooperative breeders, sharing with humans the capacity to form social bonds, develop extended families, and engage in bi-parental care (Carter et al., 1995; Carter & Keverne, 2002; Getz et al., 1981). A focus on the causes and consequences of sociality in this species offers a powerful model for studying the mechanisms through which both negative and positive social experiences influence behavior, emotion, and cardiovascular regulation.
Recent research employing prairie voles has focused on the behavioral, cardiovascular, and neurobiological consequences of long-term social isolation from their family members (versus pairing with a sibling). Chronic social isolation from a sibling induces behaviors relevant to depression in this species, consistent with behavioral changes observed in the CMS model of depression (as discussed above) and those described in human depression (American Psychiatric Association, 2000). For example, 4 weeks of social isolation in female prairie voles leads to the reduced consumption of a sucrose solution (anhedonia), and also produces defective coping strategies in a forced swimming stressor (increased immobility; learned helplessness) (Grippo et al., 2007b; Grippo et al., 2008). Similar behavioral changes in the forced swimming stressor have been observed in male prairie voles that have been temporarily separated from a female partner (Bosch et al., 2009).
Social isolation in female prairie voles also induces progressive cardiac disturbances, including increased heart rate, reduced heart rate variability, and increased cardiac arrhythmias (Grippo et al., 2007b; Grippo et al., 2010). For example, when prairie voles are exposed to a brief social crowding stressor, which involves placing the animal into a small cage with 5 strangers for 10 minutes, animals that had previously been socially isolated are more likely to experience an exaggerated heart rate response and arrhythmias versus animals that had previously been paired with a sibling (Grippo et al., 2010). These autonomic disturbances suggest that alterations of the social environment may have a profound influence on the ability of the autonomic nervous system to cope with stressors. This, in turn, can influence the progression of CVD.
Further studies with this model have shown that several cardiac changes are mediated by a withdrawal of parasympathetic tone, which may be responsible for cardiovascular morbidity and mortality associated with social isolation. This has been demonstrated by an attenuation of cardiac responsiveness to a drug that antagonizes parasympathetic receptors on the heart (atropine methyl nitrate), along with a reduction in respiratory sinus arrhythmia, which is a measure of heart rate variability that represents parasympathetic control of the heart (Grippo et al., 2007b). This withdrawal of parasympathetic tone contributes to autonomic imbalance, thereby exerting undue strain on the heart and producing changes in cardiac rate and rhythms.
Endocrine and Immune Interactions
Mechanisms of Endocrine and Immune System Interactions
The endocrine and immune systems are dysfunctional both in mood disorders and in CVD. These systems interact with the autonomic nervous system, and are directly and indirectly affected by stressors. Several components of the hypothalamic-pituitary-adrenal (HPA) axis – which is a primary stress-responsive system in the body – are disrupted in depressed patients. For example, depression has been associated with: alterations of corticotropin-releasing factor (CRF), which is released from the hypothalamus when an organism is exposed to a stressor; increases in adrenocorticotropic hormone (ACTH) or cortisol, which contribute to activation of the sympathetic nervous system; and impaired negative feedback between the HPA axis and the brain (Carroll et al., 1976; Maes et al., 1998; Raadsheer et al., 1995; Weber et al., 2000). Similar changes, reviewed elsewhere, have been observed in animal models of depression (Froger et al., 2004; Grippo et al., 2005b; Grippo et al., 2005a; Maier & Watkins, 2005). Over-activation of the HPA axis in depression therefore produces increased stress on the heart and vasculature, contributing to the pathogenesis of CVD.
The endocrine system interacts with the immune system both in the brain and the peripheral nervous system. Several lines of evidence indicate that depressive disorders are correlated with immune dysregulation (Connor & Leonard, 1998; Dantzer, 2006; Dunn et al., 2005; Hawkley et al., 2007; Kronfol, 2002; Maes, 1995; Pollak & Yirmiya, 2002; Zorrilla et al., 2001). The macrophage theory of depression (Smith, 1991) suggests that excessive secretion of immune chemicals that promote inflammation, such as cytokines and interferons, contribute to the pathophysiology of depression. For instance, when these substances are administered experimentally to humans, they induce depressive signs such as fatigue, irritability, and anorexia (Cunningham Jr. & De Souza, 1993; Niiranen et al., 1988; Spriggs et al., 1988). Also, inflammation in animals is associated with “sickness behavior” (e.g., fatigue, anhedonia, anorexia, and reduced social interactions), which is linked to the syndrome of depression (Dantzer et al., 1998; Dantzer, 2006; De La Garza II, 2005; Wichers & Maes, 2002).
Activation of the immune system is associated with specific cardiovascular disorders. Pro-inflammatory cytokines are released into the systemic circulation in the context of myocardial infarction (Das, 2000). These have adverse effects on the heart and circulation (Ferrari, 1999; Francis et al., 2004; Kapadia et al., 1998), and may act on the brain to produce signs and symptoms of depression, sickness behavior, and endocrine dysregulation. Peripheral inflammatory chemicals also act on the brain to regulate the functions of CRF and ACTH (Cassidy & O’Keane, 2000; Dunn et al., 1999), which further influences the HPA axis response to stressors, increases sympathetic drive to the heart, and creates a vicious cycle of dysfunction.
The Utility of Animal Models for Investigating Endocrine and Immune Interactions
Given the bidirectional nature of the association of mood and cardiovascular function, it is useful to study the emotional consequences of CVD using an animal model. Recent studies have focused on the behavioral sign of anhedonia in an experimental model of congestive heart failure in rats. In this model, the blood supply to the heart is restricted, causing damage (e.g., myocardial infarction, or “heart attack”). The resulting changes in the autonomic, endocrine, and immune systems mirror the syndrome of human heart failure (Felder et al., 2003; Francis et al., 2001).
Activation of the immune system during heart disease might produce a state of negative mood. Specifically, activation of the pro-inflammatory cytokine, TNF-α, has been examined as a mediator of anhedonia in rats with experimental heart failure. Circulating levels of TNF-α are increased in rats with experimental heart failure (Francis et al., 2004) and in humans with specific forms of heart disease (Levine et al., 1990). Similar to previous studies (Felder et al., 2003; Francis et al., 2003), experimental heart failure in male rats produces a significant increase in circulating TNF-α levels, and also produces a reduction in responding for rewarding electrical brain stimulation, indicative of anhedonia (Grippo et al., 2003b). However, when plasma TNF-α levels are reduced with a drug that blocks the actions of this cytokine (etanercept), the behavioral responding for rewarding electrical brain stimulation is restored to normal (control) levels, indicating a reversal of heart failure-induced anhedonia. These results suggest that heart failure can induce anhedonia via a physiological mechanism involving inflammation, and provide insight into an immune mechanism that may mediate depressive behaviors in humans with CVD. However, it is not clear exactly how circulating cytokines communicate with brain inflammatory mediators to produce changes in mood and behavior. Therefore, it is important to gain a better appreciation for central nervous system processes that regulate emotion and cardiovascular function.
Central Nervous System Dysfunction
Mechanisms Linking Mood and Cardiovascular Function in the Brain
Several central nervous system processes are altered both in mood disorders and CVD, and are affected by behavioral, physiological, or other neural inputs. Disrupted monoamine function [e.g., dopamine, norephinephrine, serotonin (5-HT)] has been implicated in depression (Lambert et al., 2000). For instance, the drug classes of monoamine oxidase inhibitors and tricyclic antidepressants, both of which influence brain monoamine levels, have been effective antidepressants in some patients (Garlow & Nemeroff, 2004). However, as these antidepressants sometimes have cardiotoxic effects in the context of CVD (Glassman, 1998), more recent research has focused on the role of the serotonergic system in depression. As reviewed elsewhere (Berman et al., 1999; Cryan et al., 2005; Lucki, 1998; Maes & Meltzer, 1995; Nalivaiko, 2006), 5-HT plays a significant role in behaviors that are disrupted in depression (e.g, mood, sleep, and appetite); depression is associated with changes in 5-HT levels and 5-HT receptors; and medications that alter 5-HT (e.g., 5-HT reuptake inhibitors) are effective antidepressants.
Central 5-HT also interacts with endocrine, immune, and autonomic function to influence cardiovascular regulation. The hypothalamic paraventricular nucleus receives serotonergic innervation, thereby affecting HPA axis functions; and this area sends projections to several brain regions that influence sympathetic and parasympathetic outflow to the cardiovascular system (Badoer, 2001; Swanson & Sawchenko, 1980). Altered 5-HT levels have been found in the brains of rodents with myocardial ischemia, and alterations of a specific 5-HT transporter gene (involved in the reuptake of 5-HT) has been associated with a higher risk of myocardial infarction in males who survived an initial infarction (Fumeron et al., 2002; Sole et al., 1983).
Aside from 5-HT, the neuropeptides oxytocin (OT) and arginine vasopressin (AVP) have been receiving increased attention in the context of emotion and autonomic function. OT and AVP play a central role in a complex neuroendocrine network that coordinates social behaviors and neurobiological responses to stressors (Amico et al., 2004; Carter, 1998; Carter & Altemus, 2005; Neumann, 2002; Porges, 2007; Taylor et al., 2006). These peptides are altered in depressed patients; while AVP has been consistently increased, it is not clear whether OT’s importance is due to an increase or a decrease of this peptide (Carter & Altemus, 2005; Purba et al., 1996; van Londen et al., 2001). OT may be released from the hypothalamus to compensate for dysfunctional endocrine and autonomic responses to stressors; however more research is required to validate this hypothesis. Interestingly, 5-HT reuptake inhibitors result in increased OT and reduced AVP secretion (see Carter & Altemus, 2005).
OT and AVP also regulate several processes that influence both emotion and cardiovascular regulation, depending on their functions in various brain regions (Neumann, 2002). OT can down-regulate the HPA axis and corresponding emotional reactions to stressors in humans and rats (Heinrichs et al., 2003; Legros et al., 1987; Windle et al., 1997). OT also has several peripheral actions that influence cardiovascular function, including beneficial effects on blood pressure and autonomic balance, and possibly reducing inflammation associated with myocardial infarction (Holst et al., 2002; Jankowski et al., 2010; Michelini et al., 2003). These neuropeptides deserve more attention in experimental studies focused on mood and cardiovascular regulation.
The Utility of Animal Models for Studying Central Nervous System Dysfunction
Pharmacological evidence indicates that 5-HT is important in the regulation of emotions and cardiovascular regulation. For example, treatment with the 5-HT reuptake inhibitor fluoxetine (Prozac) not only is an effective antidepressant, but may also improve heart rate variability in depressed individuals (Khaykin et al., 1998). However, the treatment of depressed patients with 5-HT-altering drugs does not always result in improved cardiovascular function. Findings from studies with humans have yielded inconsistent results (Dawood et al., 2007; de Jonge et al., 2010; Khaykin et al., 1998; Nemeroff et al., 1998; Roose et al., 1998), and therefore additional experimental research is necessary.
The CMS model has been used to investigate the role of 5-HT in the context of mood and cardiovascular function. The effects of fluoxetine on behavioral and cardiovascular consequences of CMS in rats have been studied by administering this 5-HT reuptake inhibitor to animals exposed to CMS. Compared to saline administration (control solution), fluoxetine fully prevents the behavioral consequences of CMS (e.g., anhedonia), but only partially prevents the cardiovascular consequences in this model (Grippo et al., 2006). For example, 4 weeks of fluoxetine, administered concurrently with 4 weeks of CMS, led to a partial (but not complete) reduction in heart rate and sympathetic drive relative to the control group. These findings suggest that reducing depressive signs and symptoms with traditional therapies may not necessarily reduce the underlying cardiovascular pathophysiology associated with this condition. These results also highlight the need for investigating the role of 5-HT receptors in the context of stress, emotion, and cardiovascular regulation (see also Nalivaiko, 2006; Sullivan Hanley & Van de Kar, 2003).
In addition to using CMS to understand the role of 5-HT in depression and heart disease, studies with the prairie vole model will provide important insight into the role of neuropeptides in mediating these conditions. Previous research has demonstrated that chronic social isolation in female prairie voles is associated with increased circulating OT and activation of OT neurons in the hypothalamic paraventricular nucleus (Grippo et al., 2007a). The influence of OT in this brain area has particular importance in the context of emotion and cardiovascular function, as information regarding stress, emotion, and autonomic regulation of the heart is integrated here (Sullivan Hanley & Van de Kar, 2003; Swanson & Sawchenko, 1980).
OT may be activated and released from the hypothalamus to compensate for altered neuroendocrine and autonomic regulation in prairie voles and humans as a result of disrupted established social bonds. Evidence for this hypothesis is derived from additional studies focusing on the potential therapeutic effects of OT in prairie voles exposed to chronic social isolation. Daily administration of OT during a period of 4 weeks of isolation in female prairie voles is protective against many of the detrimental behavioral and cardiac effects of isolation, including anhedonia, increased heart rate, and reduced parasympathetic tone (Grippo et al., 2007c). Related evidence suggest that OT has antidepressant properties (Arletti & Bertolini, 1987), and may modulate behavioral responses to short-term separation in rats (Insel & Wintink, 1991). However, as with 5-HT, additional studies will be required to understand the precise role of OT in mediating depression and cardiovascular dysregulation.
Concluding Remarks and Recommendations for Future Research
The comorbidity of depression and CVD is a significant worldwide public health problem. In combination with findings from human samples, an emphasis on biopsychosocial processes using valid, reliable, and relevant animal models offers novel advantages for the study of mechanisms underlying this association. Integrative studies that incorporate multi-system and multi-species analyses, such as those described here, enhance our understanding of the link between emotion and cardiovascular regulation by providing greater insight into both causal and common mechanisms. Along with continued mechanistic research, additional benefits will be derived from strategies that encourage dialog among researchers in varying scientific fields, including clinical, social and experimental psychology, cardiology, and neuroscience (see also Suls & Bunde, 2005). Comparative studies conducted by interdisciplinary teams, such as those described by Vaidya et al. (2004) and Willner et al. (1998), support the utility of parallel analyses in humans and animal models. These combined efforts will lead to enhanced treatment strategies, such as a greater emphasis on patient-focused therapy (see Callus et al., 2010), and will improve the quality of life for individuals with psychological and cardiovascular conditions.
Acknowledgments
The author is grateful for invaluable support from C. Sue Carter, A. Kim Johnson, Stephen W. Porges, and Louis D. Van de Kar. Financial support has been provided by: United States Public Health Service grants MH65839, MH73233, and MH77581; the American Psychological Association; and the Northern Illinois University Graduate College and Center for Biochemical and Biophysical Studies.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Amico JA, Mantella RC, Vollmer RR, Li X. Anxiety and stress responses in female oxytocin deficient mice. Journal of Neuroendocrinology. 2004;16:319–324. doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neuroscience and Biobehavioral Reviews. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sciences. 1987;41:1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clinical and Experimental Pharmacology and Physiology. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Bairey Merz CN, Dwyer J, Nordstrom CK, Walton KG, Salerno JW, Schneider RH. Psychosocial stress and cardiovascular disease: pathophysiological links. Behavioral Medicine. 2002;27:141–147. doi: 10.1080/08964280209596039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Palanza P, Costoli T, Savani E, Laviola G, Parmigiani S, et al. Chronic psychosocial stress persistently alters autonomic function and physical activity in mice. Physiology and Behavior. 2003;80:57–67. doi: 10.1016/s0031-9384(03)00209-9. [DOI] [PubMed] [Google Scholar]
- Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, et al. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? Journal of Hypertension. 2007;25:2117–2124. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Melchior M, Chastang JF, Niedhammer I, Leclerc A, Goldberg M. Social integration and mortality: a prospective study of French employees of Electricity of France-Gas of France: the GAZEL Cohort. American Journal of Epidemiology. 2004;159:167–174. doi: 10.1093/aje/kwh020. [DOI] [PubMed] [Google Scholar]
- Berman RM, Belanoff JK, Charney DS, Schatzberg AF. Principles of the pharmacotherapy of depression. In: Charney DS, Nestler EJ, Bunney BS, editors. Neurobiology of mental illness. New York: Oxford University Press; 1999. pp. 419–432. [Google Scholar]
- Bosch OJ, Nair HP, Ahern TH, Neumann ID, Young LJ. The CRF system mediates increased passive stress-coping behavior following the loss of a bonded partner in a monogamous rodent. Neuropsychopharmacology. 2009;34:1406–1415. doi: 10.1038/npp.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspectives in Biology and Medicine. 2003;46(Suppl 3):S39–S52. [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, et al. Loneliness and health: potential mechanisms. Psychosomatic Medicine. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Callus E, Quadri E, Chessa M. Elements of psychocardiology in the psychosocial handling of adults with congenital heart disease. Frontiers in Psychology. 2010;1:1–6. doi: 10.3389/fpsyg.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biological Psychiatry. 2003;54:241–247. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Rich MW, Smith LJ, Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. American Journal of Medicine. 1993;95:23–28. doi: 10.1016/0002-9343(93)90228-h. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosomatic Medicine. 2000;62:639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Carney RM, Saunders RD, Freedland KE, Stein P, Rich MW, Jaffe AS. Association of depression with reduced heart rate variability in coronary artery disease. American Journal of Cardiology. 1995;76:562–564. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. II. discrimination of depressed from nondepressed patients. Archives of General Psychiatry. 1976;33:1051–1058. doi: 10.1001/archpsyc.1976.01770090041003. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Oxytocin, vasopressin and depression. In: den Boer JA, George MS, Ter Horst GJ, editors. Current and future developments in psychopharmacology. Amsterdam: Benecke N.I.; 2005. pp. 201–216. [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience and Biobehavioral Reviews. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Keverne EB. The neurobiology of social affiliation and pair bonding. Hormones, Brain and Behavior. 2002;1:299–337. [Google Scholar]
- Cassidy EM, O’Keane V. Depression and interferon-alpha therapy. British Journal of Psychiatry. 2000;176:494. doi: 10.1192/bjp.176.5.494-a. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Leonard BE. Depression, stress, and immunological activation: the role of cytokines in depressive disorders. Life Sciences. 1998;62:583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Slattery DA. Animal models of mood disorders: recent developments. Current Opinion in Psychiatry. 2007;20:1–7. doi: 10.1097/YCO.0b013e3280117733. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and Biobehavioral Reviews. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, De Souza EB. Interleukin 1 receptors in the brain and endocrine tissues. Immunology Today. 1993;14:171–176. doi: 10.1016/0167-5699(93)90281-o. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Neurologic Clinics. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthé RM, Gheusi G, Cremona S, Layé S, Parnet P, et al. Molecular basis of sickness behavior. Annals of the New York Academy of Sciences. 1998;856:132–138. doi: 10.1111/j.1749-6632.1998.tb08321.x. [DOI] [PubMed] [Google Scholar]
- Das UN. Free radicals, cytokines and nitric oxide in cardiac failure and myocardial infarction. Molecular and Cellular Biochemistry. 2000;215:145–152. doi: 10.1023/a:1026579422132. [DOI] [PubMed] [Google Scholar]
- Dawood T, Lambert EA, Barton DA, Laude D, Elghozi JL, Esler MD, et al. Specific serotonin reuptake inhibition in major depressive disorder adversely affects novel markers of cardiac risk. Hypertension Research. 2007;30:285–293. doi: 10.1291/hypres.30.285. [DOI] [PubMed] [Google Scholar]
- de Jonge P, Rosmalen JGM, Kema IP, Doornbos B, van Melle JP, Pouwer F, et al. Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: A critical review of the literature. Neuroscience and Biobehavioral Reviews. 2010;35:84–90. doi: 10.1016/j.neubiorev.2009.11.025. [DOI] [PubMed] [Google Scholar]
- De La Garza R., II Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neuroscience and Biobehavioral Reviews. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neuroscience and Biobehavioral Reviews. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission: comparison with the effects of stress. In: Dantzer R, Wollmann EE, Yirmiya R, editors. Cytokines, stress, and depression. New York: Kluwer Academic/Plenum; 1999. pp. 117–127. [DOI] [PubMed] [Google Scholar]
- Eng PM, Rimm EB, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. American Journal of Epidemiology. 2002;155:700–709. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- Esler M, Kaye D. Sympathetic nervous system activation in essential hypertension, cardiac failure and psychosomatic heart disease. Journal of Cardiovascular Pharmacology. 2000;35(Suppl 4):S1–S7. doi: 10.1097/00005344-200000004-00001. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney D. Mood disorders and medical illness: a major public health concern. Biological Psychiatry. 2004;54:177–180. doi: 10.1016/s0006-3223(03)00639-5. [DOI] [PubMed] [Google Scholar]
- Felder RB, Francis J, Zhang ZH, Wei SG, Weiss RM, Johnson AK. Heart failure and the brain: new perspectives. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2003;284:R259–R276. doi: 10.1152/ajpregu.00317.2002. [DOI] [PubMed] [Google Scholar]
- Ferrari R. The role of TNF in cardiovascular disease. Pharmacological Research. 1999;40:97–105. doi: 10.1006/phrs.1998.0463. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Johnson AK, Felder RB. Central mineralocorticoid receptor blockade decreases plasma TNF-α after coronary artery ligation in rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2003;284:R328–R335. doi: 10.1152/ajpregu.00376.2002. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2001;281:R1734–R1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- Francis J, Zhang ZH, Weiss RM, Felder RB. Neural regulation of the proinflammatory cytokine response to acute myocardial infarction. American Journal of Physiology-Heart and Circulatory Physiology. 2004;287:H791–H797. doi: 10.1152/ajpheart.00099.2004. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Archives of General Psychiatry. 2003;60:627–636. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- Frazer A, Morilak DA. What should animal models of depression model? Neuroscience and Biobehavioral Reviews. 2005;29:515–523. doi: 10.1016/j.neubiorev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Miller GE, Sheps DS. The great debate, revisited. Psychosomatic Medicine. 2006;68:179–184. doi: 10.1097/01.psy.0000209374.25201.4e. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosomatic Medicine. 2003;65:119–128. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C, et al. Neurochemical and behavioral alterations in glucocorticoid receptor-impaired transgenic mice after chronic mild stress. Journal of Neuroscience. 2004;24:2787–2796. doi: 10.1523/JNEUROSCI.4132-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Nicaud V, Evans A, Kee F, Ruidavets JB, et al. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Temoins de l’Infarctus du Myocarde (ECTIM) Circulation. 2002;105:2943–2945. doi: 10.1161/01.cir.0000022603.92986.99. [DOI] [PubMed] [Google Scholar]
- Garlow SJ, Nemeroff CB. The neurochemistry of mood disorders: clinical studies. In: Charney DS, Nestler EJ, editors. Neurobiology of mental illness. 2. New York: Oxford University Press; 2004. pp. 440–460. [Google Scholar]
- Getz LL, Carter CS, Gavish L. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair bonding. Behavioral Ecology and Sociobiology. 1981;8:189–194. [Google Scholar]
- Glassman AH. Depression and cardiovascular comorbidity. Dialogues in Clinical Neuroscience. 2007;9:9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH. Cardiovascular effects of antidepressant drugs: updated. International Clinical Psychopharmacology. 1998;13(Suppl 5):S25–S30. doi: 10.1097/00004850-199809005-00006. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiology and Behavior. 2003a;78:703–710. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biological Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiology and Behavior. 2005a;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Weiss RM, Felder RB, Johnson AK. Cytokine mediation of experimental heart failure-induced anhedonia. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2003b;284:R666–R673. doi: 10.1152/ajpregu.00430.2002. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007a;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biological Psychiatry. 2007b;62:1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Porges SW, Carter CS. 2007 Abstract Viewer/Itinerary Planner (pp. Program No. 84.9 (Online)) Washington DC: Society for Neuroscience; 2007c. Oxytocin prevents detrimental cardiac effects of social isolation in monogamous prairie voles. [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, et al. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. American Journal of Physiology-Heart and Circulatory Physiology. 2004;286:H619–H626. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Sgoifo A, Mastorci F, McNeal N, Trahanas DM. Cardiac dysfunction and hypothalamic activation during a social crowding stressor in prairie voles. Autonomic Neuroscience: Basic and Clinical. 2010;156:44–50. doi: 10.1016/j.autneu.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology. 2005b;179:769–780. doi: 10.1007/s00213-004-2103-4. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Wu KD, Hassan I, Carter CS. Social isolation in prairie voles induces behaviors relevant to negative affect: toward the development of a rodent model focused on co-occurring depression and anxiety. Depression and Anxiety. 2008;25:E17–E26. doi: 10.1002/da.20375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm TE, Jr, Kaplan JR, Clarkson TB, Bullock BC. Effects of gender and social behavior on the development of coronary artery atherosclerosis in cynomolgus macaques. Atherosclerosis. 1983;48:221–233. doi: 10.1016/0021-9150(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Bosch JA, Engeland CG, Marucha PT, Cacioppo JT. Loneliness, dysphoria, stress, and immunity: a role for cytokines. In: Plotnikoff NP, Faith RE, Murgo AJ, Good RA, editors. Cytokines: stress and immunity. Vol. 2. Boca Raton, FL: CRC Press; 2007. pp. 67–85. [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biological Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Holst S, Uvnäs-Moberg K, Petersson M. Postnatal oxytocin treatment and postnatal stroking of rats reduce blood pressure in adulthood. Autonomic Neuroscience: Basic and Clinical. 2002;99:85–90. doi: 10.1016/s1566-0702(02)00134-0. [DOI] [PubMed] [Google Scholar]
- Insel TR, Wintink AJ. Central administration of oxytocin modulates the infant rat’s response to social isolation. European Journal of Pharmacology. 1991;203:149–152. doi: 10.1016/0014-2999(91)90806-2. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Bissonauth V, Gao L, Gangal M, Wang D, Danalache B, et al. Anti-inflammatory effect of oxytocin rat myocardial infarction. Basic Research in Cardiology. 2010;105:205–218. doi: 10.1007/s00395-009-0076-5. [DOI] [PubMed] [Google Scholar]
- Johnson AK, Anderson EA. Stress and arousal. In: Cacioppo JT, Tassinary LG, editors. Principles of psychophysiology: physical, social, and inferential elements. Cambridge: Cambridge University Press; 1990. pp. 216–252. [Google Scholar]
- Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. American Heart Journal. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- Kapadia S, Dibbs Z, Kurrelmeyer K, Karla D, Seta Y, Wang F, et al. The role of cytokines in the failing human heart. Cardiology Clinics. 1998;16:645–656. doi: 10.1016/s0733-8651(05)70041-2. [DOI] [PubMed] [Google Scholar]
- Kaplan GA, Salonen JT, Cohen RD, Brand RJ, Syme SL, Puska P. Social connections and mortality from all causes and from cardiovascular disease: prospective evidence from eastern Finland. American Journal of Epidemiology. 1988;128:370–380. doi: 10.1093/oxfordjournals.aje.a114977. [DOI] [PubMed] [Google Scholar]
- Katz R. Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacology, Biochemistry and Behavior. 1982;16:965–968. doi: 10.1016/0091-3057(82)90053-3. [DOI] [PubMed] [Google Scholar]
- Khaykin Y, Dorian P, Baker B, Shapiro C, Sandor P, Mironov D, et al. Autonomic correlates of antidepressant treatment using heart-rate variability analysis. Canadian Journal of Psychiatry. 1998;43:183–186. doi: 10.1177/070674379804300209. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Berman DS, Gransar H, Wong ND, Miranda-Peats R, White MD, et al. Social network and coronary artery calcification in asymptomatic individuals. Psychosomatic Medicine. 2005;37:343–352. doi: 10.1097/01.psy.0000161201.45643.8d. [DOI] [PubMed] [Google Scholar]
- Kristal-Boneh E, Raifel M, Froom P, Ribak J. Heart rate variability in health and disease. Scandinavian Journal of Work Environment and Health. 1995;21:85–95. doi: 10.5271/sjweh.15. [DOI] [PubMed] [Google Scholar]
- Krittayaphong R, Cascio WE, Light KC, Sheffield D, Golden RN, Finkel JB, et al. Heart rate variability in patients with coronary artery disease: differences in patients with higher and lower depression scores. Psychosomatic Medicine. 1997;59:231–235. doi: 10.1097/00006842-199705000-00004. [DOI] [PubMed] [Google Scholar]
- Kronfol Z. Immune dysregulation in major depression: a critical review of existing evidence. International Journal of Neuropsychopharmacology. 2002;5:333–343. doi: 10.1017/S1461145702003024. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. The Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Lambert G, Johansson M, Ågren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Archives of General Psychiatry. 2000;57:787–793. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- Legros JJ, Chiodera P, Geenen V, von Frenckell R. Confirmation of the inhibitory influence of exogenous oxytocin in cortisol and ACTH in man: evidence of reproducibility. Acta Endocrinologia. 1987;114:345–349. doi: 10.1530/acta.0.1140345. [DOI] [PubMed] [Google Scholar]
- Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. New England Journal of Medicine. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biological Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin A, Bonaccorso S, van Hunsel F, Van Gastel A, Delmeire L, et al. Increased 24-hour uninary cortisol excretion in patients with post-traumatic stress disorder and patients with major depression, but not in patients with fibromyalgia. Acta Psychiatrica Scandinavica. 1998;98:328–335. doi: 10.1111/j.1600-0447.1998.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY. The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. pp. 933–944. [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Updated projections of global mortality and burden of disease, 2002–2030: data sources, methods and results. Geneva: World Health Organization; 2005. [Google Scholar]
- Matthews K, Christmas D, Swan J, Sorrell E. Animal models of depression: navigating through the clinical fog. Neuroscience and Biobehavioral Reviews. 2005;29:503–513. doi: 10.1016/j.neubiorev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Marcelo MC, Amico J, Morris M. Oxytocinergic regulation of cardiovascular function: studies in oxytocin-deficient mice. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2003;284:H2269–H2276. doi: 10.1152/ajpheart.00774.2002. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the "kindling" hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychological Review. 2005;112:417–445. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Mössner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Müller N, et al. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World Journal of Biological Psychiatry. 2007;8:141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E. 5-HT1A receptors in stress-induced cardiac changes: a possible link between mental and cardiac disorders. Clinical and Experimental Pharmacology and Physiology. 2006;33:1259–1264. doi: 10.1111/j.1440-1681.2006.04521.x. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Musselman DL, Evans DL. Depression and cardiac disease. Depression and Anxiety. 1998;8(Suppl 1):71–79. [PubMed] [Google Scholar]
- Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothamo-pituitary-adrenal axis. Progress in Brain Research. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Niiranen A, Laaksonen R, Iivanainen M, Mattson K, Fäkkilä M, Cantell K. Behavioral assessment of patients treated with alpha-interferon. Acta Psychiatrica Scandinavica. 1988;78:622–626. doi: 10.1111/j.1600-0447.1988.tb06395.x. [DOI] [PubMed] [Google Scholar]
- Penninx BWJH, Beekman ATF, Honig A, Deeg DJH, Schoevers RA, van Eijk JTM, et al. Depression and cardiac mortality: results from a community-based longitudinal study. Archives of General Psychiatry. 2001;58:221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, Massari F, et al. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. American Heart Journal. 2001;141:765–771. doi: 10.1067/mhj.2001.114806. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for ‘depression due to a general medical condition’, immunotherapy and antidepressive treatment. International Journal of Neuropsychopharmacology. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Archives of General Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, van Heerikhuize JJ, Lucassen PJ, Hoogendijk WJG, Tilders FJH, Swaab DF. Corticotropin-releasing hormone mRNA levels in the paraventricular nucleus of patients with Alzheimer’s disease and depression. American Journal of Psychiatry. 1995;152:1372–1376. doi: 10.1176/ajp.152.9.1372. [DOI] [PubMed] [Google Scholar]
- Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, et al. Social engagement and the risk of cardiovascular disease mortality: results of a prospective population based study of older men. Annals of Epidemiology. 2008;18:476–483. doi: 10.1016/j.annepidem.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT, Jr, Pollock BG, et al. Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. Journal of the American Medical Association. 1998;279:287–291. doi: 10.1001/jama.279.4.287. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Linke SE, Olson MB, Francis J, Johnson BD, Bittner V, et al. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosomatic Medicine. 2008;70:282–287. doi: 10.1097/PSY.0b013e3181656e09. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, et al. Social networks are associated with lower mortality rates among women with suspected coronary disease: the National Heart, Lung, and Blood Institute sponsored Women’s Ischemia Syndrome Evaluation study. Psychosomatic Medicine. 2004;66:882–888. doi: 10.1097/01.psy.0000145819.94041.52. [DOI] [PubMed] [Google Scholar]
- Sanders BJ, Lawler JE. The borderline hypertensive rat (BHR) as a model for environmentally-induced hypertension: a review and update. Neuroscience and Biobehavioral Reviews. 1992;16:207–217. doi: 10.1016/s0149-7634(05)80181-2. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Gerin W, Davidson KW, Pickering TG, Brosschot JF, Thayer JF, et al. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosomatic Medicine. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- Seligman MEP. Depression and learned helplessness. In: Friedman RJ, Katz MM, editors. The psychology of depression: contemporary theory and research. Washington, D.C.: V.H. Winston; 1974. pp. 83–125. [Google Scholar]
- Sgoifo A, Pozzato C, Costoli T, Manghi M, Stilli D, Ferrari PF, et al. Cardiac autonomic responses to intermittent social conflict in rats. Physiology and Behavior. 2001;73:343–349. doi: 10.1016/s0031-9384(01)00455-3. [DOI] [PubMed] [Google Scholar]
- Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. Ilar Journal. 2004;45:189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- Shively CA, Musselman DL, Willard SL. Stress, depression, and coronary artery disease: modeling comorbidity in female primates. Neuroscience and Biobehavioral Reviews. 2009;33:133–144. doi: 10.1016/j.neubiorev.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosomatic Medicine. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Medical Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Sole MJ, Versteeg DH, de Kloet ER, Hussain N, Lixfeld W. The identification of specific serotonergic nuclei inhibited by cardiac vagal afferents during acute myocardial ischemia in the rat. Brain Research. 1983;265:55–61. doi: 10.1016/0006-8993(83)91333-1. [DOI] [PubMed] [Google Scholar]
- Spriggs DR, Sherman ML, Michie H, Arthor KA, Imamura K, Wilmore D, et al. Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase 1 and pharmacology study. Journal of the National Cancer Institute. 1988;80:1039–1044. doi: 10.1093/jnci/80.13.1039. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Whitehead DL. Depression, stress, and coronary heart disease: the need for more complex models. Heart. 2005;91:419–420. doi: 10.1136/hrt.2004.045310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan Hanley NR, Van de Kar LD. Serotonin and the neuroendocrine regulation of the hypothalamic-pituitary-adrenal axis in health and disease. Vitamins and Hormones. 2003;66:189–255. doi: 10.1016/s0083-6729(03)01006-9. [DOI] [PubMed] [Google Scholar]
- Suls J, Bunde J. Anger, anxiety and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Research Reviews. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. Psychosomatic Medicine. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Thurston RC, Kubzansky LD. Women, loneliness, and incident coronary heart disease. Psychosomatic Medicine. 2009;71:836–842. doi: 10.1097/PSY.0b013e3181b40efc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya JG, Grippo AJ, Johnson AK, Watson D. A comparative developmental study of impulsivity in rats and humans: the role of reward sensitivity. Annals of the New York Academy of Sciences. 2004;1021:395–398. doi: 10.1196/annals.1308.051. [DOI] [PubMed] [Google Scholar]
- Van der Kooy K, van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. International Journal of Geriatric Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, Kerkof GA, Zwinderman KH, Wiegant VM, De Wied D. Weak 24-h periodicity of body temperature and increased plasma vasopressin in melancholic depression. European Neuropsychopharmacology. 2001;11:7–14. doi: 10.1016/s0924-977x(00)00124-3. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. American Heart Journal. 2002;143:460–466. doi: 10.1067/mhj.2002.120404. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. American Heart Journal. 1999;137:453–457. doi: 10.1016/s0002-8703(99)70491-6. [DOI] [PubMed] [Google Scholar]
- Weber B, Lewicka S, Deuschle M, Colla M, Vecsei P, Heuser I. Increased diurnal plasma concentrations of cortisone in depressed patients. Journal of Clinical Endocrinology and Metabolism. 2000;85:1133–1136. doi: 10.1210/jcem.85.3.6469. [DOI] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. International Journal of Neuropsychopharmacology. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Willner P. The chronic mild stress procedure as an animal model of depression: valid, reasonably reliable, and useful. Psychopharmacology. 1997;134:371–377. [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural- neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, et al. "Depression" increases "craving" for sweet rewards in animal and human models of depression and craving. Psychopharmacology. 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- Willner P, Lappas S, Cheeta S, Muscat R. Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology. 1994;115:454–462. doi: 10.1007/BF02245568. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosomatic Medicine. 2003;65:201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behavior and Immunity. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]