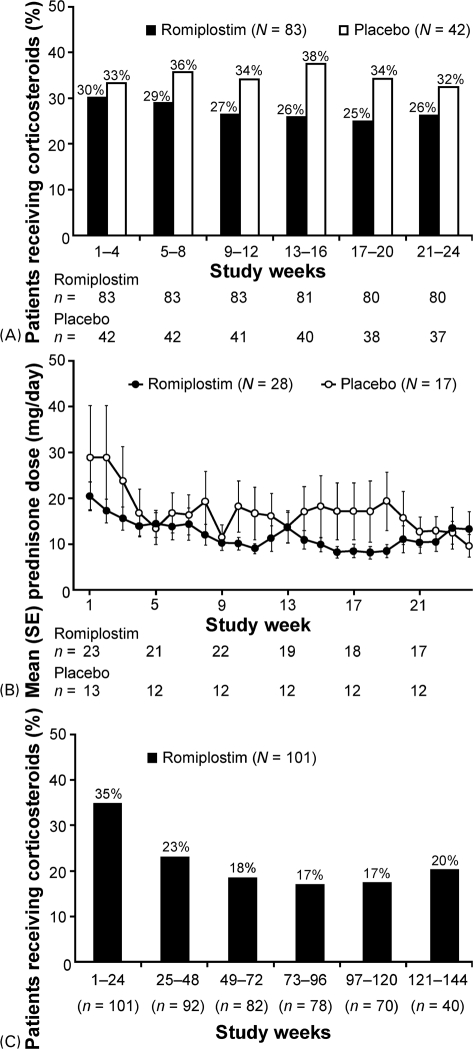
Figure 2.
(A) Corticosteroid use decreased in romiplostim-treated patients during two, 6-month, phase III studies. Percentages calculated from n = number of subjects remaining on study at the start of the relevant time period. (B) Prednisone dose decreased in patients continuing to receive corticosteroids during the phase III studies. N = Number of subjects receiving prednisone-type corticosteroids during the phase III studies; n = number of subjects receiving prednisone-type corticosteroids at specified time point. (C) Corticosteroid use decreased significantly over time in patients treated with romiplostim for up to 3 years in an open-label extension study. n = number of subjects remaining on study.