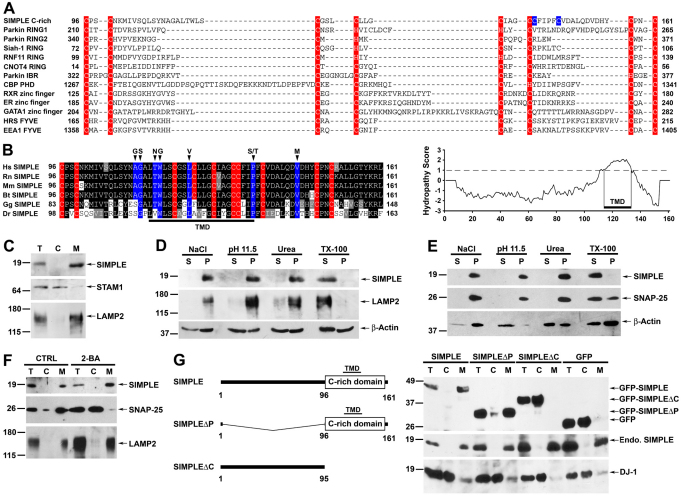
Fig. 2.
SIMPLE is a transmembrane protein that requires its cysteine-rich (C-rich) domain for membrane association. (A) Sequence alignment of the SIMPLE C-rich domain with known RING finger domains and other C-rich domains. Residues implicated in zinc binding are highlighted in red, and two unaligned SIMPLE cysteine residues are colored in blue. (B) SIMPLE is predicted to be a single-spanning integral membrane protein. Cysteine residues are highlighted in red and residues mutated in CMT1C are highlighted in blue. Hydropathy plot (TMpred) indicates the presence of transmembrane domain (TMD) within the SIMPLE C-rich domain. (C) Post-nuclear supernatant (T) from HeLa cells was separated into cytosol (C) and membrane (M) fractions. Aliquots representing an equal percentage of each fraction were subjected to immunoblot analysis with antibodies against SIMPLE, STAM1 and LAMP2. (D,E) Membrane fractions from HeLa cells (D) or SH-SY5Y cells (E) were subjected to extraction by 1.5 M NaCl, 0.1 M Na2CO3 (pH 11.5), 4 M urea or 1% Triton X-100 (TX-100) and were then separated into supernatant (S) and pellet (P) fractions. Aliquots representing an equal percentage of each fraction were analyzed by immunoblotting with antibodies against SIMPLE, LAMP2 (D), SNAP-25 (E) and β-actin. (F) The membrane association of SIMPLE is palmitoylation independent. Post-nuclear supernatants of SH-SY5Y cells treated with 100 μM 2-bromohexadeconic acid (2-BA) or the vehicle control (CTRL) were separated into cytosol (C) and membrane (M) fractions. Aliquots representing an equal percentage of each fraction were subjected to immunoblot analysis with antibodies against SIMPLE, SNAP-25 and LAMP2. (G) Domain structures of SIMPLE and its deletion mutants (left). Post-nuclear supernatants (T) of HeLa cells expressing GFP-tagged SIMPLE, SIMPLEΔP, SIMPLEΔC or GFP were separated into cytosol (C) and membrane (M) fractions (right). Aliquots representing an equal percentage of each fraction were analyzed by immunoblotting with antibodies against GFP, SIMPLE and DJ-1.