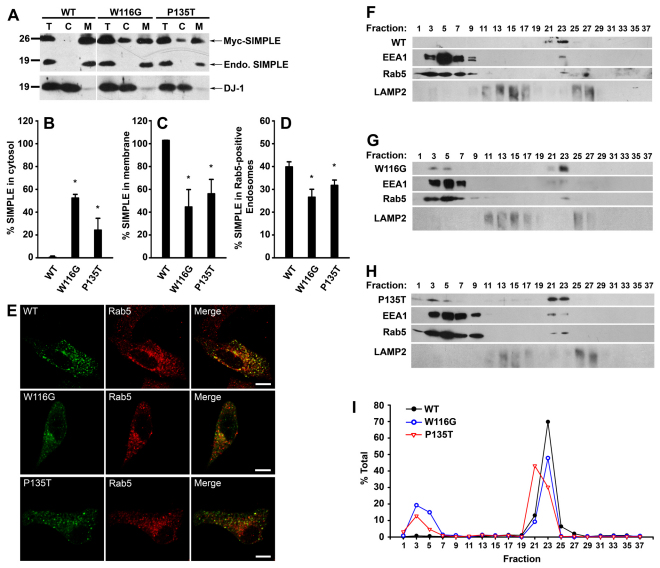
Fig. 4.
CMT1C-associated SIMPLE mutants are mislocalized from the early endosomal membrane to the cytosol. (A) Post-nuclear supernatants (T) from HeLa cells expressing Myc-tagged WT, W116G or P135T SIMPLE proteins were separated into cytosol (C) and membrane (M) fractions. Aliquots representing an equal percentage of each fraction were analyzed by immunoblotting with anti-DJ-1 antibody and anti-SIMPLE antibody, which recognized both Myc-tagged and endogenous (endo.) SIMPLE proteins. (B,C) The percentages of SIMPLE WT or mutant proteins in the cytosol (B) and membrane (C) fraction relative to the total amount in the corresponding post-nuclear supernatant (T) were quantified and shown as mean ± s.e.m. from three independent experiments. *P<0.05 compared with SIMPLE WT. (D) The percentages of SIMPLE WT or mutant proteins localized to Rab5-positive early endosomes in transfected HeLa cells were quantified as described in the Materials and Methods. Data represent mean ± s.e.m. from three independent experiments. *P<0.05 compared with SIMPLE WT. (E) Representative images from D. HeLa cells expressing Myc-tagged SIMPLE WT or mutant proteins were immunostained with antibodies against the Myc tag (green) and the early endosome marker Rab5 (red). Colocalization is indicated by the yellow color in the merged panels. Scale bars: 10 μm. (F–H) Post-nuclear supernatants from stably transfected HEK293 cells expressing Myc-tagged WT (F), W116G (G) and P135T (H) SIMPLE were fractionated on a 10–30% linear Optiprep gradient into 38 fractions, with fraction 1 corresponding to the top of the gradient. Equal volumes of each fraction were subjected to SDS-PAGE followed by immunoblot analysis. The lower band in the EEA1 immunoblots represents a EEA1 protein degradation product. (I) The level of SIMPLE WT or mutant proteins in each fraction was quantified and is shown as a percentage of the total level of the protein. Data are representative of at least three independent experiments.