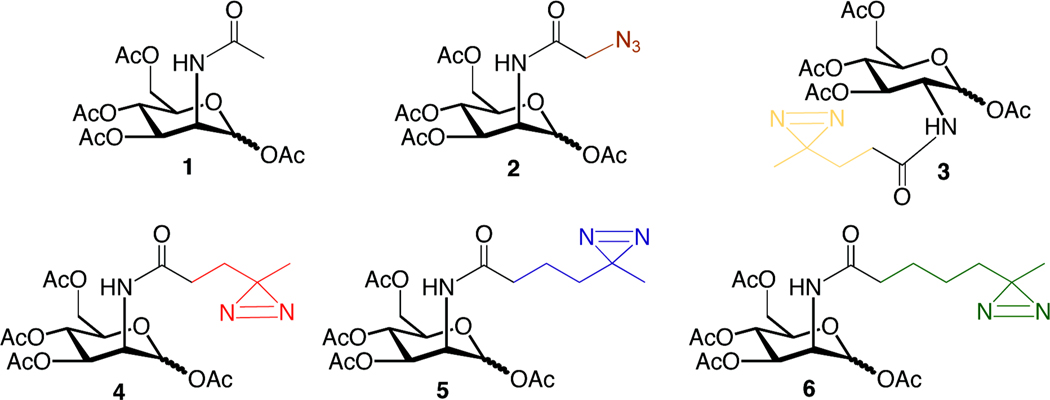
Figure 1. Per-O-acetylated N-acyl hexosamines.
The N-acyl side chains of ManNAc and N-acetylglucosamine (GlcNAc) analogs were modified by the addition of a diazirine or an azide. Ac4ManNAc (1) is a protected form of naturally occurring ManNAc. Ac4ManNAz (2) is an azide-containing analog that is metabolized to sialosides with high efficiency 24. The names of the diazirine-modified sugars (3–6) are derived from the number of methylene groups separating the carbonyl and diazirine on the N-acyl side chain: Ac4GlcNDAz(2me) (3), Ac4ManNDAz(2me) (4), Ac4ManNDAz(3me) (5), and Ac4ManNDAz(4me) (6).