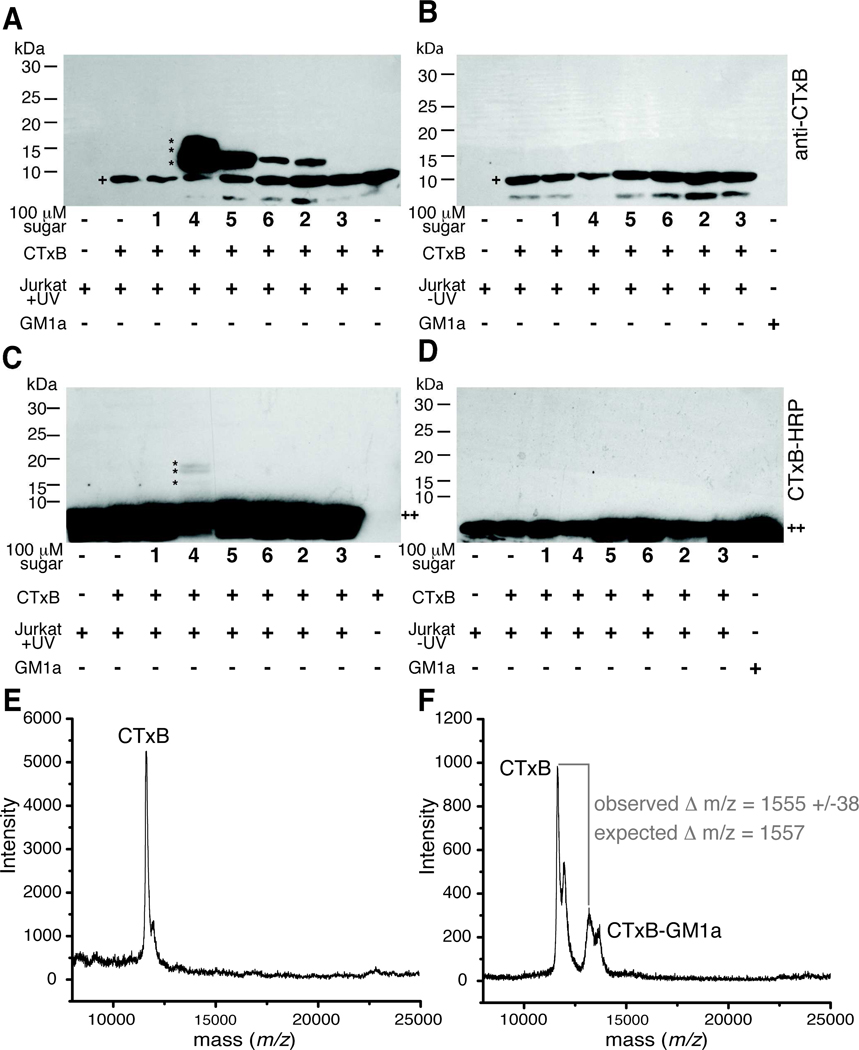
Figure 7. Photocrosslinking sugar analogs covalently capture CTxB-GM1a interaction.
Jurkat cells were cultured with or without sugar analogs 1–6 for 72 hours and then incubated with CTxB for 45 min at 4 °C. Cells were photoirradiated, lysed, and the lysates separated by reducing SDS-PAGE followed by protein transfer to PVDF membranes. UV-irradiated (A) and non-UV-irradiated samples (B) were probed for the presence of CTxB (+) using an anti-CTxB antibody. A slower mobility band has an apparent molecular weight consistent with formation of a CTxB-GM1a complex (~13 kDa, *). The identity of the very low molecular weight band (<10 kDa) is not known, but it appears to be related to a contaminant in the CTxB preparation. UV-irradiated (C) and non-UV-irradiated samples (D) were probed for the presence of GM1a (++) using CTxB-HRP. A slower mobility band has an apparent molecular weight consistent with formation of a CTxB-GM1a complex (~13 kDa, *). Membranes were stripped and probed for β–actin to demonstrate equal protein loading in each lane (not shown). (E) SAMDI-TOF MS spectrum of SAM-bound monomeric CTxB species derived from cells cultured with Ac4ManNAc, incubated with CTxB, and photoirradiated. (F) SAMDI-TOF MS spectrum of SAM-bound monomeric CTxB and crosslinked CTxB-GM1a-SiaDAz(2me) derived from cells cultured with Ac4ManNDAz(2me), incubated with CTxB, and photoirradiated.