Abstract
Objective
Knowledge remains limited regarding cerebral blood flow autoregulation after cardiac arrest and during post-resuscitation hypothermia. We determined the relationship of cerebral blood flow to cerebral perfusion pressure in a swine model of pediatric hypoxic-asphyxic cardiac arrest during normothermia and hypothermia and tested novel measures of autoregulation derived from near-infrared spectroscopy.
Design
Prospective, balanced animal study.
Setting
Basic physiology laboratory at an academic institution.
Subjects
Eighty-four neonatal swine.
Interventions
Piglets underwent hypoxic-asphyhxic cardiac arrest or sham surgery and recovered for 2 hours with normothermia followed by 4 hours of either moderate hypothermia or normothermia. In half of the groups, blood pressure was slowly decreased through inflation of a balloon catheter in the inferior vena cava to identify the lower limit of cerebral autoregulation at 6 hours post-resuscitation. In the remaining groups, blood pressure was gradually increased by inflation of a balloon catheter in the aorta to determine the autoregulatory response to hypertension. Measures of autoregulation obtained from standard laser-Doppler flowmetry and indices derived from near-infrared spectroscopy were compared.
Measurements and Main Results
Laser-Doppler flux was lower in post-arrest animals compared to sham-operated controls during the 2-hour normothermic period after resuscitation. During the subsequent 4-hour recovery, hypothermia decreased laser-Doppler flux in both the sham surgery and post-arrest groups. Autoregulation was intact during hypertension in all groups. With arterial hypotension, post-arrest, hypothermic piglets had a significant decrease in the perfusion pressure lower limit of autoregulation compared to post-arrest, normothermic piglets. The near-infrared spectroscopy-derived measures of autoregulation accurately detected loss of autoregulation during hypotension.
Conclusions
In a pediatric model of cardiac arrest and resuscitation, delayed induction of hypothermia decreased cerebral perfusion and decreased the lower limit of autoregulation. Metrics derived from non-invasive near-infrared spectroscopy accurately identified the lower limit of autoregulation during normothermia and hypothermia in piglets resuscitated from arrest.
Keywords: pediatrics, heart arrest, cerebrovascular circulation, ischemia, blood pressure, hypothermia
INTRODUCTION
Approximately 16,000 American children suffer a cardiac arrest annually, and many suffer severe neurologic injuries (1). Cerebrovascular autoregulation maintains relatively constant cerebral blood flow (CBF) over a range of cerebral perfusion pressure (CPP). The impact of cardiac arrest and resuscitation on autoregulation is unclear. Evidence in adult humans (2, 3) and rats (4) indicates that cardiac arrest disrupts autoregulation and shifts the lower limit of autoregulation (LLA) to a higher blood pressure (3). Data from an experimental pediatric model also suggests that autoregulation becomes impaired after arrest (4). Treatment guidelines for pediatric cardiac arrest include considering induced hypothermia to 32–34°C for comatose patients (5). To our knowledge, the effects of post-resuscitation hypothermia on autoregulation have not been investigated in immature brain.
We developed methods to continuously monitor autoregulation using near infrared spectroscopy (NIRS): the cerebral oximetry (COx) and hemoglobin volume (HVx) indices. COx is calculated by a moving, linear correlation coefficient representing the relationship between cerebral oximetry and blood pressure and is based on the assumption that changes in tissue oxygen saturation are proportional to changes in CBF over brief periods with stable cerebral metabolic rate. Passivity of cerebral oximetry to blood pressure indicates loss of autoregulation (6–8). HVx is a moving, linear correlation coefficient between relative total tissue hemoglobin (rTHb) and blood pressure. HVx is based on the assumption that autoregulatory vasodilation/vasoconstriction produce changes in cerebral blood volume that are proportional to changes in rTHb. Passivity of rTHb to mean arterial blood pressure (MAP) indicates loss of vasoreactivity (9). Compared to laser-Doppler flowmetry, COx and HVx accurately identify the LLA in swine (6–9). The utility of COx and HVx monitoring after pediatric cardiac arrest has not been explored.
In immature brain, the range of autoregulation is limited and appears to increase as MAP increases during development (10–13). In neonatal piglets, CBF is autoregulated over a MAP range of 50–90 mmHg. Significant decreases in CBF have been reported at MAP below 40 mmHg whereas significant increases are apparent when MAP is increased to 100–120 mmHg (14–16). We examined the effects of hypoxic-asphyxic cardiac arrest and moderate hypothermia on autoregulation in neonatal swine. We hypothesized that cardiac arrest disrupts autoregulation and that hypothermia preserves autoregulation. We further postulated that HVx and COx accurately reflect autoregulation after arrest.
MATERIALS AND METHODS
Subjects
All procedures were approved by the Animal Care and Use Committee at Johns Hopkins University, and care and handling of the animals were in accord with National Institutes of Health Guidelines. Neonatal male piglets (3–5 days old, 1–2.5 kg) underwent hypoxic-asphyxic cardiac arrest or sham surgery. Animals were divided into groups that were exposed to normothermia or hypothermia during recovery. Piglets were further divided into subgroups to undergo induced hypotension or hypertension. Thus, eight groups were studied (hypotensive cohorts: post-arrest normothermia, post-arrest hypothermia, sham normothermia, and sham hypothermia; hypertensive cohorts: post-arrest normothermia, post-arrest hypothermia, sham normothermia, and sham hypothermia).
Animal Preparation
Anesthesia was induced with 5% isoflurane with 50/50 nitrous oxide/oxygen. Animals were intubated and mechanically ventilated. Femoral arteries and veins were cannulated, and anesthesia was maintained with fentanyl (10 mcg/kg + 10–20 mcg/kg/hr, IV), vecuronium (1 mg/kg/hr), 50/50 nitrous oxide/oxygen, and 1.5% isoflurane. Isoflurane was decreased to 0.8% after surgery. Piglets received D5½NS at 10 cc/hr. Three craniotomy sites were made for: (1) an extra-ventricular drain catheter for ICP monitoring, (2) a 1-mm diameter laser-Doppler flow (LDF) probe (Moor Instruments, Devon, U.K.; model DRT4; 60 Hz), and (3) a temperature probe in the epidural space. The LDF probe was positioned in frontoparietal cortex 5 mm from the ICP monitor and secured with rubber washers and cemented to the skull. A NIRS probe (Somanetics, Troy, MI; light emitting diode to shallow and deep photodiode distances of 30 mm and 40 mm, respectively) was placed over frontoparietal cortex contralateral to the craniotomy sites.
Hypoxic-asphyxic cardiac arrest
The fractional inspired oxygen concentration (FiO2) was decreased to 10% for 45 mins. Then, 5 mins of room air was supplied to briefly re-oxygenate the heart. This brief re-oxygenation period is required for successful cardiac resuscitation. The endotracheal tube was then occluded for 7 mins. Piglets were resuscitated with 100% oxygen, manual chest compressions, and epinephrine (100 mcg/kg, IV). After resuscitation, the FiO2 was decreased to 50% to maintain oxyhemoglobin saturation > 93%, and sodium bicarbonate and calcium chloride were administered for metabolic acidosis and hypocalcemia. Sham-operated piglets received the same anesthesia, surgery, and duration of anesthesia, but without arrest. They were ventilated with an FiO2 of 50% throughout the experiment.
Temperature
After resuscitation, piglets in the normothermia groups were maintained at target rectal and brain temperatures of 38.5°C (normothermic for swine) with warming blankets and heating lamps. Piglets in the hypothermia groups first underwent 2 hrs of normothermia (designed to mimic the potential delay in initiating hypothermia in clinical practice) and then had hypothermia induced to 32°C with an external cooling blanket for the remainder of the experiment. Ice packs were placed on the head and body during the first 30 mins of hypothermia to facilitate cooling. Approximately 30 mins is required to decrease rectal temperature to 32°C, which has been found to track brain temperature within 0.2°C in this model (17). Animals in all groups remained anesthetized and received muscle relaxants and ventilator support.
LDF-derived autoregulation monitoring
At 6 hrs of return of spontaneous circulation (ROSC), blood pressure was gradually decreased or increased in the designated cohorts. To determine the LLA, we decreased MAP over a 3-hr span by inflating a 5-Fr balloon catheter in the inferior vena cava (IVC) (7). To examine the autoregulatory response to hypertension, we increased MAP over 3 hrs by inflating an aortic balloon catheter and infusing phenylephrine and dopamine. To assess CBF, cortical red blood cell (RBC) flux was measured with the LDF probe. MAP, ICP, and LDF measurements were sampled from an analog-to-digital converter at 100 Hz with ICM+ software (Cambridge University, Cambridge, UK). CPP was calculated as MAP – ICP and recorded every 10 secs. The autoregulation responses to hypo- or hypertension were examined by plotting LDF as a function of CPP. The LLA was identified by dichotomizing the data such that the fit of two linear regression lines results in the lowest combined error squared. The CPP at the intersection of these two lines was defined as the LLA (6, 7, 9). The autoregulatory response to hypertension was evaluated by calculating the static rate of autoregulation (sROR), which is the percent change in cerebrovascular resistance (CVR) divided by the percent change in CPP (%ΔCVR/ %ΔCPP). An sROR less than 0.4 indicates impaired autoregulation, and an sROR of 1 represents perfect autoregulation (18, 19).
NIRS-derived autoregulation indices (COx, HVx)
Cerebral oximetry and rTHb were synchronously sampled from the digital output of the NIRS monitor at a refresh rate of 30/min and filtered as previously described (6, 9). COx was calculated by a continuous moving Pearson correlation between cerebral oximetry and CPP, and HVx was similarly calculated from rTHb and MAP (6, 7, 9). These indices display a biphasic pattern. When autoregulation is functional, COx and HVx are near-zero or negative (because CBF/CBV and CPP are not positively correlated). When autoregulation becomes impaired with hypotension, the indices become positive, indicating pressure-passive CBF and CBV. HVx and COx were compared to LDF-derived measures of autoregulation.
Statistical analysis
Data are presented as means ± S.D. unless otherwise stated. Differences were considered significant at p ≤0.05. Statistical analyses were performed with STATA (v11.1), SigmaStat 3.1 (SYSTAT) and Microsoft Excel. For physiologic parameters not expected to be affected by temperature or arrest, one-way ANOVA was performed on the data or ranks based on tests of normality and equal variance. Subsequent multiple comparison tests (Newman-Keuls or post-hoc Dunn’s) were performed as needed. Two-way ANOVAs were performed to examine the effects of arrest and temperature on LDF, LLA, and sROR followed by appropriate post-hoc comparisons. Spline regression analyses were performed to evaluate biphasic models of the COx and HVx responses to hypotension. Receiver operator characteristic (ROC) curves were calculated to test the sensitivity and specificity of COx and HVx in detecting CPP above or below the LDF-derived LLA. Both the spline regression and ROC analyses accounted for multiple intra-subject measurements. For the ROC curves, CPP was divided into 5-mmHg bins and coded as a binary variable above or below the bin containing the LLA, and COx and HVx were analyzed as continuous variables (−1 to +1). Linear regression analysis was used to evaluate HVx and COx during induced hypertension. Post-hoc power analyses were performed with nQuery Advisor (v7) and SAS (v9.2) for differences in LLA.
RESULTS
Eighty-four piglets underwent cardiac arrest or sham surgery. The resuscitation rate was 78% (9 of 41 could not be resuscitated). Eleven animals were excluded from the study owing to technical problems, including intracranial hemorrhage during placement of the ICP monitor and problems with the LDF signal. In the hypotensive cohort, technical problems occurred in three sham and two arrest piglets. In the hypertensive cohort, technical problems occurred in three sham and three arrest normothermic piglets. Data were analyzed on 64 piglets (32 arrests and 32 shams; n=8 per group). Post-hoc power analysis using the contrast between the lowest and highest of the four LLA means (31 and 41 mmHg) among the four hypotensive groups (normothermic and hypothermic sham, normothermic and hypothermic post-arrest) indicated a power of detecting a difference at alpha 0.05 as 74%.
LDF analysis from combined hypotensive and hypertensive cohorts prior to blood pressure manipulation
Post-arrest piglets had lower LDF than sham piglets during the first 2 hrs of normothermia after ROSC. Sham animals had LDF that was 97±8% of baseline (n=32), whereas post-arrest piglets had LDF that was 85±20% of pre-arrest baseline (n=32; p<0.001 between groups). The PaCO2 values at baseline, ROSC 1 hr, and ROSC 2 hr did not differ among sham or post-arrest piglets. In post-arrest piglets, the PaCO2 was 39±4 mmHg at baseline, 38±6 mmHg at ROSC 1 hr, and 40±4 mmHg at ROSC 2 hr (p=0.195; n=32). The PaCO2 at equivalent times in sham piglets was 40±7 mmHg at baseline, 41±6 mmHg at ROSC 1 hr, and 40±7 mmHg at ROSC 2 hr (p=0.692; n=32).
Between 2 and 6 hrs of recovery, both arrest (p=0.006) and hypothermia (p<0.001) significantly decreased LDF without an interaction effect (p=0.159). LDF was 94±12% of baseline in normothermic shams (n=16), 76±14% of baseline in hypothermic shams (n=16), 87±23% of pre-arrest baseline in normothermic post-arrest piglets (n=16), and 57±21% of pre-arrest baseline in hypothermic post-arrest piglets (n=16; Figure 1).
Figure 1.
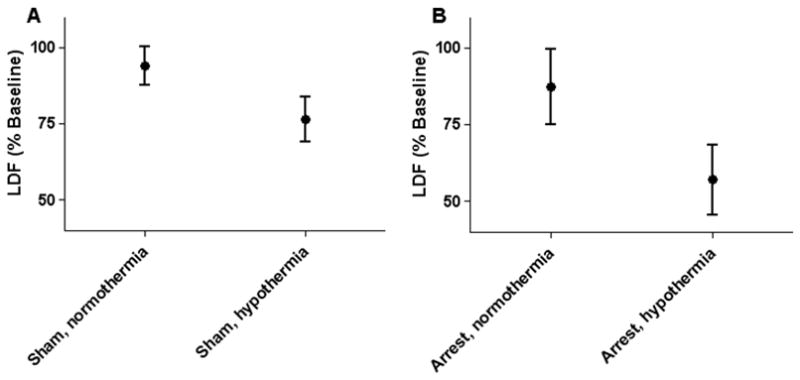
Both arrest (p = 0.006) and hypothermia (p < 0.001) significantly decreased laser-Doppler flux (LDF) without interaction effect. (A) Normothermic and hypothermic sham animals (n = 16 per group). (B) Normothermic and hypothermic post-arrest animals (n = 16 per group). Data are presented as means with 95% confidence intervals.
Hypotensive cohorts
CPP and ICP were not different among groups during the 0–2 hrs after ROSC when all animals remained normothermic (ROSC 0–2 hrs), and during the subsequent 4 hrs of normothermia or hypothermia (ROSC 2–6 hrs) prior to induced hypotension (Table 1). Brain and body temperature also were not different until hypothermia was induced.
Table 1.
Physiologic variables of piglets in hypotensive groups.
| Parameter a | Group (n=8) | Baseline | Hypoxia 42 mins | Asphyxia 7 mins | ROSC 0–2 hrs | ROSC 2–6 hrs |
|---|---|---|---|---|---|---|
| CPP (mmHg) | NT arrest | 58±5 | 34±18 | 22±10 | 52±8 | 49±7 |
| HT arrest | 59±6 | 36±16 | 22±9 | 46±7 | 46±7 | |
| NT sham | 58±9 | N/A | N/A | 53±11 | 48±6 | |
| HT sham | 65±7 | N/A | N/A | 61±9 | 53±8 | |
| ICP (mmHg) | NT arrest | 7±6 | 13±8 | 8±4 | 6±4 | 9±3 |
| HT arrest | 6±5 | 9±8 | 5±3 | 7±3 | 10±3 | |
| NT sham | 7±3 | N/A | N/A | 8±4 | 7±5 | |
| HT sham | 5±4 | N/A | N/A | 7±5 | 6±5 | |
| Body temp (°C) | NT arrest | 37.7±1.0 | 38.2±0.6 | 37.9±0.6 | 38.6±0.7 | 39.0±0.8 |
| HT arrest | 37.3±0.6 | 38.1±0.9 | 37.5±1.2 | 38.6±0.5 | 31.7±1.0 | |
| NT sham | 37.8±1.4 | N/A | N/A | 38.5±1.0 | 38.8±0.5 | |
| HT sham | 37.6±0.8 | N/A | N/A | 38.9±0.8 | 32±0.9 | |
| Brain temp (°C) | NT arrest | 37.4±0.6 | 37.8±0.7 | 36.7±0.6 | 38.0±1.0 | 38.5±0.7 |
| HT arrest | 36.5±1.0 | 37.3±1.3 | 36.1±1.4 | 37.6±0.9 | 31.7±0.7 | |
| NT sham | 37.1±1.4 | N/A | N/A | 37.9±0.7 | 38.3±0.7 | |
| HT sham | 37.2±1.1 | N/A | N/A | 38.5±0.9 | 31.7±0.8 |
CPP, cerebral perfusion pressure; HT, hypothermic; ICP, intracranial pressure; NT, normothermic; ROSC, return of spontaneous circulation; N/A, not applicable.
As expected, asphyxia produced severe hypoxia, hypercapnea, and acidosis (Table 2). All groups showed restoration of arterial pH, PaCO2, and PaO2. Although some statistical differences were detected among groups, these differences were not considered physiologically large. PaCO2 was somewhat higher during hypothermia when corrected for body temperature (p<0.05). Sodium, potassium, chloride, and ionized calcium levels were not different among groups (data not shown), with the exception of sodium levels at ROSC 6 hrs where hypothermic shams had sodium of 146±5 mEq/L and normothermic shams had sodium of 139±2 mEq/L (p<0.05).
Table 2.
Blood gas and hemoglobin levels of piglets in hypotensive groups.
| Parametera | Group (n=8) | Baseline | Hypoxia 42 mins | Asphyxia 5 mins | ROSC 1 hrs | ROSC 3 hrs | ROSC 6 hrs |
|---|---|---|---|---|---|---|---|
| pH | NT arrest | 7.45±0.04 | 7.29±0.07 | 6.88±0.11 | 7.48±0.06 | 7.48±0.05 | 7.51±0.06 |
| HT arrest | 7.45±0.05 | 7.32±0.05 | 6.84±0.08 | 7.38±0.13 | 7.51±0.08 | 7.42±0.04* | |
| NT sham | 7.45±0.05 | N/A | N/A | 7.44±0.05 | 7.43±0.06 | 7.44±0.05 | |
| HT sham | 7.46±0.10 | N/A | N/A | 7.44±0.05 | 7.44±0.09 | 7.37±0.05* | |
| PaCO2 | NT arrest | 41±5 | 42±3 | 92±18 | 35±7 | 44±4 | 39±5 |
| HT arrest | 41±3 | 43±7 | 109±16 | 43±4* | 40±7 | 50±7* | |
| NT sham | 38±5 | N/A | N/A | 40±6 | 40±6 | 38±2 | |
| HT sham | 37±7 | N/A | N/A | 40±5 | 38±7 | 48±5* | |
| PaO2 | NT arrest | 187±76 | 24±4 | 12±5 | 220±47 | 213±42 | 210±40 |
| HT arrest | 207±58 | 25±5 | 10±5 | 223±50 | 246±42 | 228±67 | |
| NT sham | 225±38 | N/A | N/A | 217±26 | 222±19 | 231±22 | |
| HT sham | 216±39 | N/A | N/A | 217±42 | 265±38 | 262±32 | |
| Hb | NT arrest | 7.0±1.3 | 7.5±1.6 | 6.7±1.1 | 6.6±2.2 | 7.0±0.8 | 6.1±1.2 |
| HT arrest | 6.3±1.3 | 6.9±0.9 | 7.7±1.9 | 6.7±0.4 | 6.8±1.2 | 7.5±1.8 | |
| NT sham | 6.1±1.4 | N/A | N/A | 6.3±1.9 | 5.9±2.0 | 5.6±1.6 | |
| HT sham | 6.7±1.6 | N/A | N/A | 7.6±1.3 | 7.3±1.4 | 7.8±1.7* |
Temperature-corrected blood gases presented during hypothermia. Hb, hemoglobin; HT, hypothermic; NT, normothermic; ROSC, return of spontaneous circulation; N/A, not applicable.
p<0.05 from normothermic group.
Cerebrovascular autoregulation during hypotension
The LLA was similar between normothermic and hypothermic sham groups (38±8 mmHg and 39±9 mmHg, respectively). The LLA was lower in the hypothermic, post-arrest group (31±6 mmHg) in comparison to that of piglets resuscitated from arrest with normothermia (41±6 mmHg) (p=0.012). There was no significant difference in the LLA between normothermic sham and normothermic post-arrest piglets (Figure 2).
Figure 2.
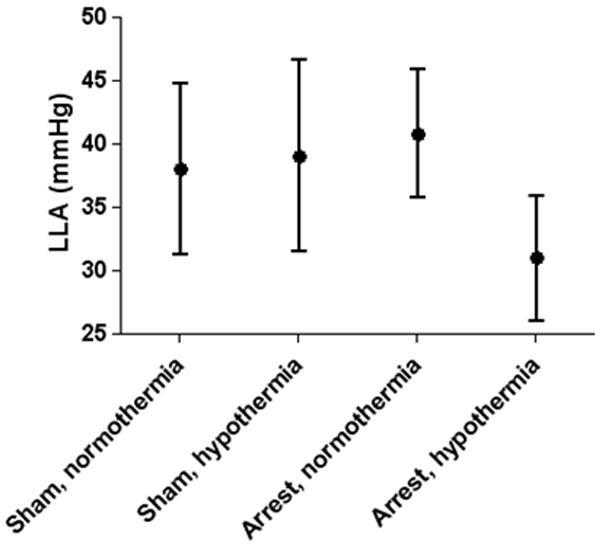
The cerebral perfusion pressure lower limit of autoregulation (LLA) was significantly lower in piglets after hypoxic-asphyxic cardiac arrest with post-resuscitation hypothermia compared to sham-operated piglets and piglets resuscitated from cardiac arrest with normothermia (p = 0.012). Data are presented as means with 95% confidence intervals.
HVx and COx increased as CPP was decreased, and the slope of the relationship with CPP was significantly less than zero (Table 3). However, in some groups, the relationship was biphasic with the slope below the LLA significantly more negative than the slope above the LLA (Table 3, Figures 3 and 4). For HVx, these groups included normothermic sham and normothermic arrested piglets. For COx, this occurred in normothermic arrested piglets. Based on the area under the ROC curves (Table 4), HVx and COx were good predictors of whether CPP was above or below the LLA, although accuracy was slightly less in post-arrest animals. For normothermic and hypothermic sham piglets, there was a 50% probability that CPP exceeded the LLA at HVx 0.22 and COx 0.41. For normothermic and hypothemic post-arrest piglets, there was a 50% probability that CPP exceeded the LLA at HVx 0.21 and COx 0.42.
Table 3.
Results of spline regression analyses of the relationships of hemoglobin volume (HVx) and cerebral oximetry (COx) indices with cerebral perfusion pressure (CPP) during induced hypotension. Slopes of the relationships of the indices vs. CPP below the lower limit of autoregulation (LLA) are shown in the left-hand columns along with significance compared to a slope of zero. Differences in the slopes of the relationships of HVx and COx with CPP below the LLA from the slopes above the LLA are shown in the right-hand columns.
| Group a | Slope at CPP Below LLA | (Slope Above LLA) − (Slope Below LLA) | |||
|---|---|---|---|---|---|
| Slope (95% C.I. b) | p-value | Difference (95% C.I.) | p-value | ||
| NT sham | HVx | −0.019 (−0.034, −0.004) | 0.014 | 0.019 (0.003, 0.036) | 0.022 |
| COx | −0.011(−0.011,−0.001) | 0.075 | 0.020 (−0.001, 0.042) | 0.059 | |
| HT sham | HVx | −0.016 (−0.031, −0.001) | 0.033 | −0.003 (−0.022, 0.015) | 0.731 |
| COx | −0.017 (−0.025, −0.010) | <0.001 | −0.001 (−0.011, 0.009) | 0.819 | |
| NT arrest | HVx | −0.050 (−0.062, −0.037) | <0.001 | 0.030 (0.009, 0.051) | 0.006 |
| COx | −0.034 (−0.049, −0.020) | <0.001 | 0.037 (0.011, 0.634) | 0.006 | |
| HT arrest | HVx | −0.016 (−0.027, −0.005) | 0.005 | 0.007 (−0.008, 0.023) | 0.364 |
| COx | −0.017 (−0.030, −0.004) | 0.008 | 0.012 (−0.002,−0.027) | 0.098 | |
HT, hypothermic; NT, normothermic.
C.I., confidence interval.
Figure 3.
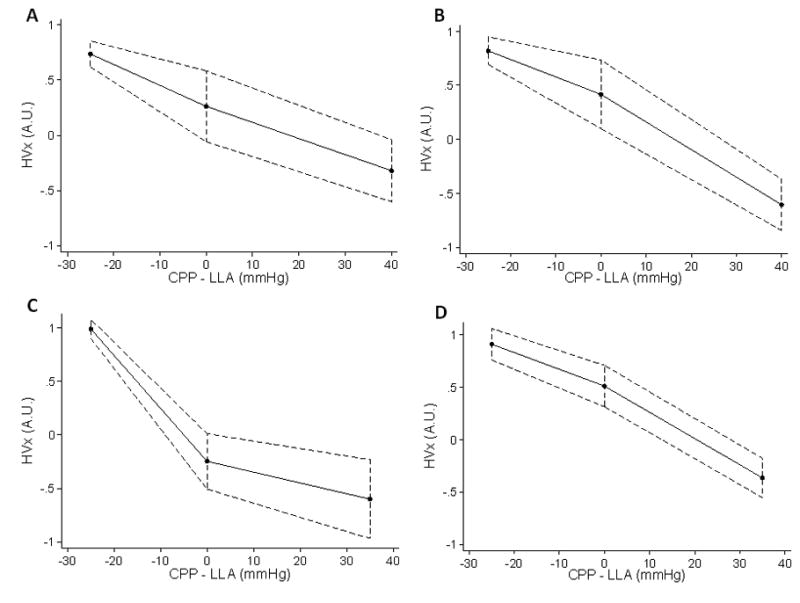
Spline regression model fit lines of the hemoglobin volume (HVx) index during induced hypotension. Each piglet’s lower limit of autoregulation (LLA) is centered to zero on the x-axis, permitting comparison of each animal’s cerebral perfusion pressure (CPP) relative to LLA. HVx became more positive as CPP decreased below the LLA in (A) normothermic sham, (B) hypothermic sham, (C) normothermic post-arrest, and (D) hypothermic post-arrest piglets. (n = 8 per group). Spline regression analysis indicated that the slope of the relationship of HVx with CPP became significantly more negative below the LLA in normothermic sham (p = 0.022) and normothermic arrested groups (p = 0.006).
Figure 4.
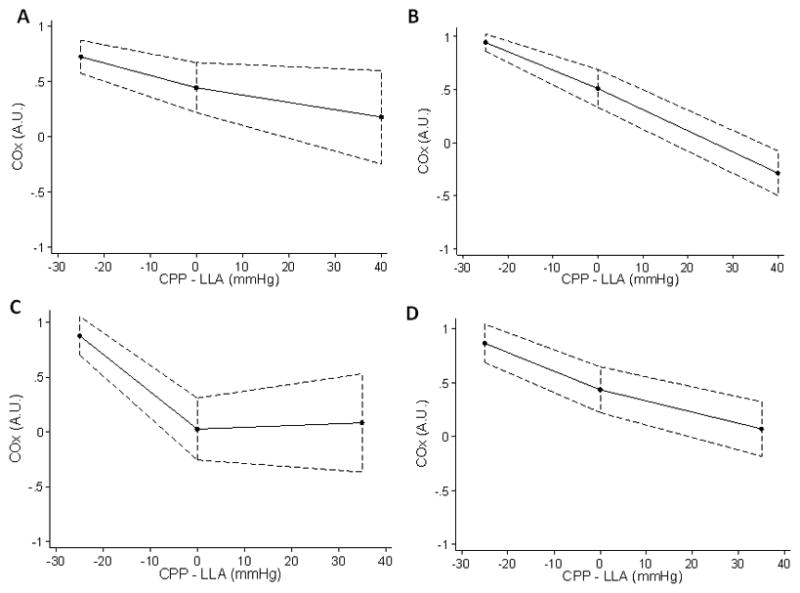
Spline regression model fit lines of the cerebral oximetry volume (COx) index during induced hypotension. Each piglet’s lower limit of autoregulation (LLA) is centered to zero on the x-axis, permitting comparison of each animal’s cerebral perfusion pressure (CPP) relative to LLA. COx became more positive as CPP decreased below the LLA in (A) normothermic sham, (B) hypothermic sham, (C) normothermic post-arrest, and (D) hypothermic post-arrest piglets. (n = 8 per group). Spline regression analysis indicated that the slope of the relationship of COx with CPP became significantly more negative below the LLA in the normothermic arrested group (p = 0.006).
Table 4.
Results of the receiver operator characteristic curves (ROC) and area under the curves (AUC) for the hemoglobin volume (HVx) and cerebral oximetry (COx) in detecting the lower limit of autoregulation (LLA) during induced hypotension.
| Group a | No. Observations (across 8 piglets per group) | AUC | |
|---|---|---|---|
| NT sham | HVx | 66 | 0.96 |
| COx | 67 | 0.94 | |
| HT sham | HVx | 78 | 0.94 |
| COx | 74 | 0.96 | |
| NT arrest | HVx | 73 | 0.87 |
| COx | 69 | 0.81 | |
| HT arrest | HVx | 69 | 0.97 |
| COx | 63 | 0.82 |
NT, normothermic; HT, hypothermic.
Hypertensive cohorts
No differences were observed in CPP or ICP among the groups in which hypertension was induced, and brain and body temperature were similar before inducing hypothermia (Table 5). Small differences were observed among groups in the recovery of arterial pH and hemoglobin concentration (Table 6). Sodium, potassium, chloride, and ionized calcium levels were not different among groups (data not shown), with the exception of sodium levels at ROSC 1 hr where hypothermic post-arrest animals had sodium of 160±14 mEq/L and normothermic post-arrest animals had sodium of 152±14 mEq/L (p<0.05).
Table 5.
Physiologic variables of piglets in hypertensive groups
| Parametera | Group (n=8) | Baseline | Hypoxia 42 mins | Asphyxia 7 mins | ROSC 0–2 hrs | ROSC 2–6 hrs |
|---|---|---|---|---|---|---|
| CPP (mmHg) | NT arrest | 61±17 | 38±16 | 21±15 | 48.1±7.0 | 43.4±4.4 |
| HT arrest | 65±15 | 37±16 | 20±8 | 50.9±11.0 | 46.1±4.5 | |
| NT sham | 65±11 | N/A | N/A | 58.8±8.5 | 56.4±9.8 | |
| HT sham | 57±12 | N/A | N/A | 57.4±11.5 | 53.0±10.0 | |
| ICP (mmHg) | NT arrest | 7±4 | 10±5 | 7±3 | 6.9±2.6 | 9.4±3.3 |
| HT arrest | 7±5 | 10±6 | 6±3 | 6.5±4.0 | 6.9±3.6 | |
| NT sham | 6±5 | N/A | N/A | 8.9±5.4 | 8.0±4.6 | |
| HT sham | 11±7 | N/A | N/A | 11.9±5.6 | 10.5±5.7 | |
| Body temp (°C) | NT arrest | 37.7±1.2 | 38.9±0.8 | 39.1±0.9 | 38.8±0.8 | 39.1±0.6 |
| HT arrest | 38.4±0.5 | 38.6±0.6 | 38.4±0.6 | 38.4±0.7 | 31.9±1.1 | |
| NT sham | 37.3±0.9 | N/A | N/A | 38.1±0.9 | 38.7±0.6 | |
| HT sham | 37.1±0.8 | N/A | N/A | 38.2±1.0 | 32.1±0.5 | |
| Brain temp (°C) | NT arrest | 36.7±1.1 | 37.8±0.8 | 37.1±0.8 | 37.6±1.0 | 38.1±0.8 |
| HT arrest | 37.5±0.5 | 37.7±1.1 | 36.6±1.1 | 37.5±0.8 | 31.8±0.7 | |
| NT sham | 36.6±1.2 | N/A | N/A | 37.4±1.0 | 37.8±0.6 | |
| HT sham | 37.0±1.3 | N/A | N/A | 38.0±1.2 | 31.7±0.5 |
CPP, cerebral perfusion pressure; HT, hypothermic; ICP, intracranial pressure; NT, normothermic; ROSC, return of spontaneous circulation; N/A, not applicable.
Table 6.
Blood gas and hemoglobin levels of piglets in hypertensive groups
| Parametera | Group (n=8) | Baseline | Hypoxia 42 mins | Asphyxia 5 mins | ROSC 1 hrs | ROSC 3 hrs | ROSC 6 hrs |
|---|---|---|---|---|---|---|---|
| pH | NT arrest | 7.49±0.05 | 7.30±0.13 | 6.83±0.11 | 7.49±0.07 | 7.47±0.09 | 7.49±0.05 |
| HT arrest | 7.44±0.02 | 7.28±0.08 | 6.82±0.05 | 7.39±0.08* | 7.47±0.06 | 7.40±0.05* | |
| NT sham | 7.46±0.09 | N/A | N/A | 7.44±0.05 | 7.43±0.05 | 7.46±0.05 | |
| HT sham | 7.45±0.06 | N/A | N/A | 7.46±0.06 | 7.42±0.05 | 7.41±0.04* | |
| PaCO2 | NT arrest | 37±3 | 43±7 | 101±17 | 37±5 | 43±5 | 40±5 |
| HT arrest | 38±3 | 42±10 | 100±17 | 38±7 | 40±4.9 | 45±8 | |
| NT sham | 41±8 | N/A | N/A | 43±8 | 44±13 | 40±4 | |
| HT sham | 42±7 | N/A | N/A | 40±6 | 42±4 | 44±5 | |
| PaO2 | NT arrest | 234±21 | 26±4 | 16±5 | 218±40 | 207±25 | 221±11 |
| HT arrest | 229±16 | 25±6 | 15±6 | 193±44 | 241±40 | 229±35 | |
| NT sham | 228±26 | N/A | N/A | 218±25 | 219±26 | 233±27 | |
| HT sham | 222±33 | N/A | N/A | 216±41 | 261±52 | 260±46 | |
| Hb | NT arrest | 6.8±1.6 | 7.9±2.3 | 8.1±2.6 | 6.4±0.8 | 6.7±1.1 | 6.3±1.6 |
| HT arrest | 8.0±1.8 | 8.2±1.7 | 8.0±1.8 | 7.3±1.0 | 8.8±2.3 | 8.9±1.6* | |
| NT sham | 7.1±2.1 | N/A | N/A | 7.7±1.9 | 7.4±1.6 | 7.1±1.3 | |
| HT sham | 6.1±2.0 | N/A | N/A | 6.6±2.6 | 7.0±2.0 | 7.2±1.6 |
Temperature-corrected blood gases presented during hypothermia. Hb, hemoglobin; HT, hypothermic; NT, normothermic; ROSC, return of spontaneous circulation; N/A, not applicable.
p<0.05 from normothermic group
Animals maintained autoregulation during the induction of hypertension (Figures 5A and 5B). CPP was increased to a maximum of 125±19 mmHg in normothermic sham piglets, 112±26 mmHg in hypothermic sham piglets, 115±22 mmHg in normothermic post-arrest piglets, and 103±14 mmHg in hypothermic post-arrest piglets. Further increases were not tolerated as cardiac failure and pulmonary edema often developed. Normothermic and hypothermic shams had sROR 0.64±0.26 and 0.47±0.28, respectively. Normothermic and hypothermic post-arrest piglets had sROR 0.59±0.28 and 0.70±0.23, respectively (p=0.466; Figure 5C). As CPP increased, values for HVx and COx did not increase above zero and were consistent with preserved autoregulation during post-resuscitation normothermia and hypothermia (Figure 6).
Figure 5.
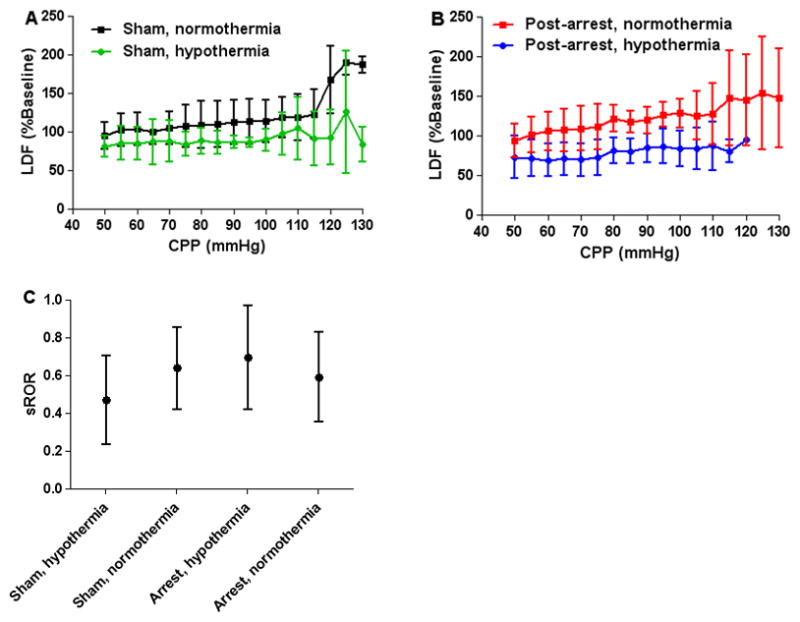
All cohorts had preserved autoregulation during hypertension. (A) Sham piglets had relatively constant laser-Doppler flux (LDF, mean ± SD) during normothermia and hypothermia (n = 8 per group). (B) Piglets resuscitated from arrest also had relatively constant LDF during normothermia and hypothermia (n = 8 per group). (C) The static rate of autoregulation (sROR, mean, 95% confidence intervals) of all groups indicated intact autoregulation during hypertension (p = 0.466).
Figure 6.
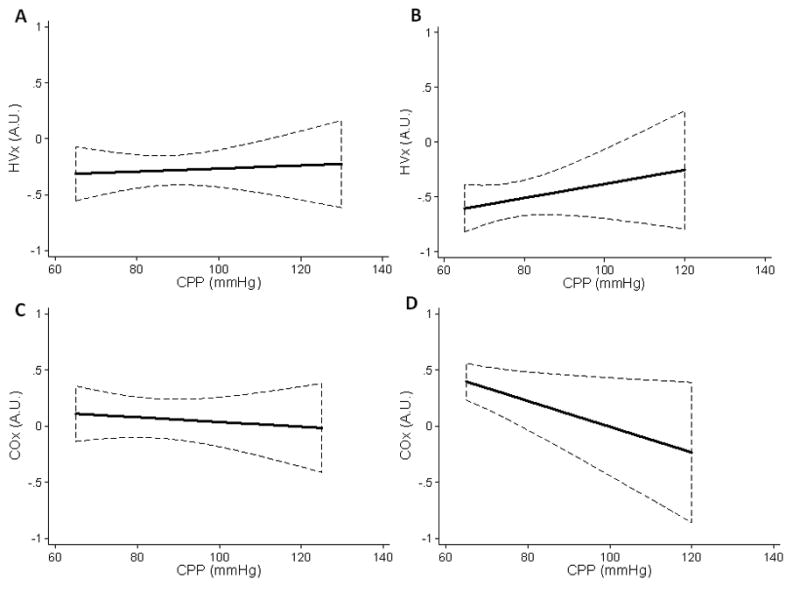
Linear regression lines show that the hemoglobin volume (HVx) and cerebral oximetry (COx) indices were consistent with preserved autoregulation during induced hypertension. (A) Post-arrest, normothermic piglets (n = 8), and (B) post-arrest, hypothermic piglets (n = 8) displayed negative HVx with rising cerebral perfusion pressure (CPP), indicating intact cerebrovascular reactivity. (C) Post-arrest, normothermic piglets (n = 8), and (D) post-arrest, hypothermic (n = 8) piglets also had overall low COx values, indicating functional autoregulation. Data are shown with 95% confidence intervals.
DISCUSSION
The hypoxia-asphyxia protocol reflects the most common etiology of pediatric cardiac arrests (20). Moreover, the pattern of regional brain injury in this piglet model (21, 22) corresponds to the selective vulnerability of primary sensorimotor cortex, striatum, and thalamus seen in newborns with hypoxic-ischemic encephalopathy (23). Here, we positioned the LDF probe over vulnerable primary sensorimotor cortex. LDF measurements permit relative changes in RBC flux to be tracked in cerebral cortex to estimate changes in CBF and have been correlated with radioisotope tracer (24) and microsphere (25) measurements of regional changes in cortical CBF. A limitation of the LDF technique is that it measures RBC flux in a small volume of tissue (approximately 1 mm3) and may be subject to variability arising from regional heterogeneity in autoregulatory behavior (26). Nevertheless, a close correspondence has been reported between the autoregulatory range determined by LDF and autoradiographic tracer at diffferent postnatal ages in rabbits (12). Moreover, we observed a close correspondence between the regional LDF measurements and the NIRS-based indices, which are derived from a much larger tissue volume. NIRS techniques have been shown to correlate with arterial spin-labeling MRI (27) and computed tomography perfusion imaging measures of CBF (28).
Given these limitations, we did not find a difference in the LDF-derived LLA between sham-operated piglets and those resuscitated from arrest with normothermic recovery. Past studies in humans (2, 3) and rats (4) demonstrated impaired autoregulation after cardiac arrest with a shift in the LLA to a higher MAP (3). It is likely that the degree of cerebral injury affects the extent of disruption in autoregulation. Although neuronal injury occurs in this model (21, 22), neuronal cell death in cerebral cortex largely evolves beyond the 6–9 hour period when autoregulation was measured in the present study. In addition, these piglets did not have substantial intracranial hypertension, which can exert an independent effect on LLA (16). Moreover, the piglets are capable of regaining consciousness in the absence of anesthesia, whereas many patients will remain unconscious at 6–9 hours after arrest. Thus, it is possible that a more severe insult or longer recovery period would reveal a shift in the LLA. We did not extend the duration of asphyxia because the ability to resuscitate the heart becomes markedly difficult.
We identified a significant decrease in the LLA in post-arrest piglets that recovered during hypothermia compared to the other three groups. This observation suggests that hypothermia may enable pressure-reactive CBF at perfusion pressures that would normally result in pressure-passivity after arrest. At normal CPP, the post-arrest hypothermic group had a nearly 50% decrease in LDF from pre-arrest baseline. Consequently, increases in baseline cerebrovascular resistance during hypothermia may also expand the range of perfusion pressures at which cerebrovascular reactivity remains intact. The slightly elevated PaCO2 in the hypothermic groups would act to increase LDF and to increase the LLA. Therefore, PaCO2 does not account for the decrease in the LLA seen in the post-arrest hypothermic group.
The LLA between normothermic and hypothermic shams did not differ. It is possible that our sample size was too small to detect a difference. Post-hoc power analysis indicated a power of detecting a difference at alpha 0.05 as 74%. Although the power values for these comparisons are lower than the traditional 80%, some differences in LLA are large enough to be detected with this sample size. However, smaller differences may not be detectable. Additional studies are needed to further evaluate the effects of hypothermia on the LLA.
Delayed decreases in CBF are often present in humans and adult animals after global cerebral ischemia. By pooling normothermic cohorts, a moderate decrease in LDF could be detected. Although there was a trend toward a small increase in ICP over 6 hrs of recovery, CPP was not significantly affected. Moreover, autoregulation was intact. Thus, the lower LDF was not secondary to low CPP.
Hypothermia showed significantly reduced LDF in post-arrest and sham animals despite similar CPP. This decrease is likely related to decreases in oxygen demand (30). Moreover, Cheng et al. (31) demonstrated decreased CBF coupled with decreased metabolic rate in a piglet model of cerebral hypoxia and hypothermia. Our results indicate that this decline in CBF also occurs when the initiation of hypothermia is delayed until 2 hrs after ROSC and remains sustained throughout 6 hrs of moderate hypothermia.
The NIRS-derived autoregulation indices detected the LLA across cohorts. CPP below the LLA was detected by index values of COx 0.41 and HVx 0.22 in sham animals and COx 0.42 and HVx 0.21 in arrested animals. This was in agreement with our previous work demonstrating optimal sensitivity and specificity at a COx threshold of 0.42 in naïve piglets (8). Our results indicate that continuous autoregulation monitoring with NIRS can identify the CPP limits at which the cerebral vasculature is most responsive to changes in CPP. This has important clinical implications and could guide clinicians in clarifying individual patient hemodynamic goals.
In unanesthetized piglets, increasing MAP to 90 mmHg by aortic balloon inflation has been reported to result in no change in CBF, whereas increases in MAP to 100–120 mmHg produced increases in CBF that were attributed to increased prostanoid production (14). In the present study on anesthetized piglets, we did not observe a consistent increase in LDF in the CPP range of 100–120 mmHg. Perhaps anesthesia reduces the release of vasodilator prostanoids during hypertension or extends the autoregulatory upper limit by effects on baseline myogenic tone. We were unable to increase CPP above 100–120 mmHg because heart failure occurred. To fully examine the autoregulatory response to hypertension in this model, more aggressive cardiopulmonary support, such as bypass, or a different anesthetic regimen that does not affect cardiac contractility may be needed. Moreover, it is unclear if our results in neonatal piglets apply to older children. Juvenile animals with a greater range of cardiac function may be required to model arrest in children older than infants.
Conclusions
Hypothermia after hypoxic-asphyxic cardiac arrest decreases CBF and decreases the LLA. Continuous autoregulation monitoring with NIRS-derived indices can identify the LLA and may be useful clinically in tailoring blood pressure management in individual patients with different degrees of autoregulatory capacity in the acute recovery period from cardiac arrest with or without hypothermia.
Acknowledgments
Supported in part by: grants from the Foundation for Anesthesia Education and Research, the American Society of Critical Care Anesthesiologists, and Hospira (Dr. Lee); NIH NS060703 (Dr. Koehler); NIH HL092259 (Dr. Hogue)
Dr. Lee received funding support from the National Institutes of Health for the Pediatric Loan Repayment Program. Dr. Brady consulted for Sommetrics, received a patent for the ICMT software and receives royalties from the patent licensing fee. Dr. Hogue received a grant from Sommetrics. Dr. Jordan is a member of the Critical Event Committee of Berlin Heart for an FDA monitored device trial. Dr. Smielewski received a share of the ICMT software licensing fee. Dr. Czosnyka consulted for Physiosonics, received honoraria/speaking fees from Integra and received royalties from ICMT Licensing. The remaining authors have not disclosed any potential conflicts of interest.
We would like to thank Carol Thompson, M.S., M.B.A. from the Johns Hopkins Bloomberg School of Public Health, Department of Biostatistics for her guidance, teaching, and assistance with the statistical analysis. Dr. Lee was a recipient of The Hartwell Foundation Biomedical Research Fellowship Award.
Dr. Lee is also a recipient of the Foundation for Anesthesia Education and Research (FAER) Research Fellowship Grant (co-sponsors the American Society of Critical Care Anesthesiologists and Hospira), the American Heart Association Scientist Development Grant, the International Anesthesia Research Society Clinical Scholar Research Award, and the National Institutes of Health (NIH) Pediatric Loan Repayment Program (NINDS). Dr. Brady was a recipient of The Hartwell Foundation Individual Biomedical Research Award and the FAER Mentored Research Training Grant. Dr. Easley was a recipient of the FAER Mentored Research Training Grant. Dr. Koehler was supported by NIH NS060703. Dr. Hogue was supported by NIH HL092259. Dr. Smielewski and Dr. Czosnyka were supported by the National Institute of Health Research, Biomedical Research Centre, Cambridge University Hospital Foundation Trust – Neurosciences Theme.
Footnotes
No reprints will be ordered.
All work was performed at Johns Hopkins University.
References
- 1.Topjian AA, Berg RA, Nadkarni VM. Pediatric cardiopulmonary resuscitation: Advances in science, techniques, and outcomes. Pediatrics. 2008 Nov;122(5):1086–98. doi: 10.1542/peds.2007-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishizawa H, Kudoh I. Cerebral autoregulation is impaired in patients resuscitated after cardiac arrest. Acta Anaesthesiol Scand. 1996 Oct;40(9):1149–53. doi: 10.1111/j.1399-6576.1996.tb05579.x. [DOI] [PubMed] [Google Scholar]
- 3.Sundgreen C, Larsen FS, Herzog TM, Knudsen GM, Boesgaard S, Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke. 2001 Jan;32(1):128–32. doi: 10.1161/01.str.32.1.128. [DOI] [PubMed] [Google Scholar]
- 4.Manole MD, Foley LM, Hitchens TK, Kochanek PM, Hickey RW, Bayir H, Alexander H, Ho C, Clark RS. Magnetic resonance imaging assessment of regional cerebral blood flow after asphyxial cardiac arrest in immature rats. J Cereb Blood Flow Metab. 2009 Jan;29(1):197–205. doi: 10.1038/jcbfm.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Liaison Committee on Resuscitation. The international liaison committee on resuscitation (ILCOR) consensus on science with treatment recommendations for pediatric and neonatal patients: Pediatric basic and advanced life support. Pediatrics. 2006 May;117(5):e955–77. doi: 10.1542/peds.2006-0206. [DOI] [PubMed] [Google Scholar]
- 6.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: Comparison of 3 methods. Stroke. 2008 Sep;39(9):2531–7. doi: 10.1161/STROKEAHA.108.514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, Koehler RC, Shaffner DH. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007 Oct;38(10):2818–25. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady KM, Mytar JO, Kibler KK, Hogue CW, Jr, Lee JK, Czosnyka M, Smielewski P, Easley RB. Noninvasive autoregulation monitoring with and without intracranial pressure in the naive piglet brain. Anesth Analg. 2010 Jul;111(1):191–5. doi: 10.1213/ANE.0b013e3181e054ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, Koehler RC, Shaffner DH, Brady KM. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009 May;40(5):1820–6. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 10.Papile LA, Rudolph AM, Heymann MA. Autoregulation of cerebral blood flow in the preterm fetal lamb. Pediatr Res. 1985 Feb;19(2):159–61. doi: 10.1203/00006450-198502000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Ramaekers VT, Casaer P, Daniels H, Marchal G. Upper limits of brain blood flow autoregulation in stable infants of various conceptional age. Early Hum Dev. 1990 Dec;24(3):249–58. doi: 10.1016/0378-3782(90)90032-e. [DOI] [PubMed] [Google Scholar]
- 12.Tuor UI, Grewal D. Autoregulation of cerebral blood flow: Influence of local brain development and postnatal age. Am J Physiol. 1994 Dec;267(6 Pt 2):H2220–8. doi: 10.1152/ajpheart.1994.267.6.H2220. [DOI] [PubMed] [Google Scholar]
- 13.Helou S, Koehler RC, Gleason CA, Jones MD, Jr, Traystman RJ. Cerebrovascular autoregulation during fetal development in sheep. Am J Physiol. 1994 Mar;266(3 Pt 2):H1069–74. doi: 10.1152/ajpheart.1994.266.3.H1069. [DOI] [PubMed] [Google Scholar]
- 14.Chemtob S, Beharry K, Rex J, Varma DR, Aranda JV. Changes in cerebrovascular prostaglandins and thromboxane as a function of systemic blood pressure. cerebral blood flow autoregulation of the newborn. Circ Res. 1990 Sep;67(3):674–82. doi: 10.1161/01.res.67.3.674. [DOI] [PubMed] [Google Scholar]
- 15.Laptook AR, Stonestreet BS, Oh W. Brain blood flow and O2 delivery during hemorrhagic hypotension in the piglet. Pediatr Res. 1983 Jan;17(1):77–80. doi: 10.1203/00006450-198301000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Czosnyka M, Smielewski P, Shaffner DH. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009 Apr;108(4):1278–83. doi: 10.1213/ane.0b013e3181964848. [DOI] [PubMed] [Google Scholar]
- 17.Agnew DM, Koehler RC, Guerguerian AM, Shaffner DH, Traystman RJ, Martin LJ, Ichord RN. Hypothermia for 24 hours after asphyxic cardiac arrest in piglets provides striatal neuroprotection that is sustained 10 days after rewarming. Pediatr Res. 2003 Aug;54(2):253–62. doi: 10.1203/01.PDR.0000072783.22373.FF. [DOI] [PubMed] [Google Scholar]
- 18.Strebel S, Lam AM, Matta B, Mayberg TS, Aaslid R, Newell DW. Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. 1995 Jul;83(1):66–76. doi: 10.1097/00000542-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Vavilala MS, Tontisirin N, Udomphorn Y, Armstead W, Zimmerman JJ, Chesnut R, Lam AM. Hemispheric differences in cerebral autoregulation in children with moderate and severe traumatic brain injury. Neurocrit Care. 2008;9(1):45–54. doi: 10.1007/s12028-007-9036-9. [DOI] [PubMed] [Google Scholar]
- 20.Moler FW, Meert K, Donaldson AE, Nadkarni V, Brilli RJ, Dalton HJ, Clark RS, Shaffner DH, Schleien CL, Statler K, Tieves KS, Hackbarth R, Pretzlaff R, van der Jagt EW, Levy F, Hernan L, Silverstein FS, Dean JM Pediatric Emergency Care Applied Research Network. In-hospital versus out-of-hospital pediatric cardiac arrest: A multicenter cohort study. Crit Care Med. 2009 Jul;37(7):2259–67. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997 Jan 13;377(2):262–85. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 22.Brambrink AM, Martin LJ, Hanley DF, Becker KJ, Koehler RC, Traystman RJ. Effects of the AMPA receptor antagonist NBQX on outcome of newborn pigs after asphyxic cardiac arrest. J Cereb Blood Flow Metab. 1999 Aug;19(8):927–38. doi: 10.1097/00004647-199908000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Miller SP, Ramaswamy V, Michelson D, Barkovich AJ, Holshouser B, Wycliffe N, Glidden DV, Deming D, Partridge JC, Wu YW, Ashwal S, Ferriero DM. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005 Apr;146(4):453–60. doi: 10.1016/j.jpeds.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Dirnagl U, Kaplan B, Jacewicz M, Pulsinelli W. Continuous measurement of cerebral cortical blood flow by laser-doppler flowmetry in a rat stroke model. J Cereb Blood Flow Metab. 1989 Oct;9(5):589–96. doi: 10.1038/jcbfm.1989.84. [DOI] [PubMed] [Google Scholar]
- 25.Bishai JM, Blood AB, Hunter CJ, Longo LD, Power GG. Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: A comparison of laser doppler and microsphere measurements of CBF. J Physiol. 2003 Feb 1;546(Pt 3):869–78. doi: 10.1113/jphysiol.2002.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones SC, Radinsky CR, Furlan AJ, Chyatte D, Qu Y, Easley KA, Perez-Trepichio AD. Variability in the magnitude of the cerebral blood flow response and the shape of the cerebral blood flow-pressure autoregulation curve during hypotension in normal rats [corrected] Anesthesiology. 2002 Aug;97(2):488–96. doi: 10.1097/00000542-200208000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Carp SA, Dai GP, Boas DA, Franceschini MA, Kim YR. Validation of diffuse correlation spectroscopy measurements of rodent cerebral blood flow with simultaneous arterial spin labeling MRI; towards MRI-optical continuous cerebral metabolic monitoring. Biomed Opt Express. 2010 Aug 10;1(2):553–65. doi: 10.1364/BOE.1.000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott JT, Diop M, Tichauer KM, Lee TY, St Lawrence K. Quantitative measurement of cerebral blood flow in a juvenile porcine model by depth-resolved near-infrared spectroscopy. J Biomed Opt. 2010 May-Jun;15(3):037014. doi: 10.1117/1.3449579. [DOI] [PubMed] [Google Scholar]
- 29.Arrich J, Holzer M, Herkner H, Mullner M. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2009 Oct 7;4(4):CD004128. doi: 10.1002/14651858.CD004128.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Busija DW, Leffler CW. Hypothermia reduces cerebral metabolic rate and cerebral blood flow in newborn pigs. Am J Physiol. 1987 Oct;253(4 Pt 2):H869–73. doi: 10.1152/ajpheart.1987.253.4.H869. [DOI] [PubMed] [Google Scholar]
- 31.Cheng G, Sun J, Wang L, Shao X, Zhou W. Effects of selective head cooling on cerebral blood flow and metabolism in newborn piglets after hypoxia-ischemia. Early Hum Dev. 2011 Feb;87(2):109–14. doi: 10.1016/j.earlhumdev.2010.11.007. [DOI] [PubMed] [Google Scholar]


