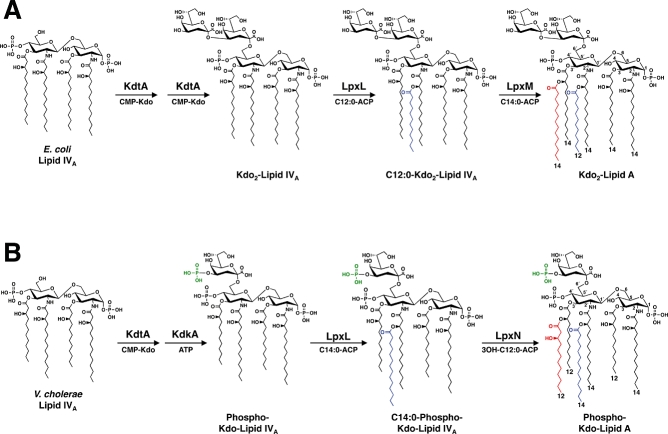
Fig. 1.
Comparison of the late stages of Kdo-lipid A biosynthesis in E. coli and V. cholerae. A. E. coli possess a bi-functional Kdo transferase (KdtA, also known as WaaA), which transfers two Kdo sugars to the lipid A precursor lipid IVA. The lipid A secondary acyltransferases, LpxL and LpxM then catalyse the transfer of a laurate (C12:0) (blue) to the 2′-position and a myristate (C14:0) (red) to the 3′-position of the glucosamine disaccharide, creating Kdo2-lipid A. B. The V. cholerae genome encodes a mono-functional KdtA, which transfers one Kdo residue to lipid IVA. The Kdo kinase (KdkA) then phosphorylates the Kdo sugar (green), resulting in phosphorylated Kdo-lipid IVA. V. cholerae LpxL (Vc0213) catalyses the transfer of a myristate (C14:0) (blue) to the 2′-position of the glucosamine disaccharide. As shown in the current work, LpxN (Vc0212) transfers a 3-hydroxylaurate (3-OH C12:0) (red) to the 3′-position, generating the hexa-acylated V. cholerae Kdo-lipid A domain. The exact order in which the secondary acyl chains are added in V. cholerae has not been determined.