Abstract
The Phox2b genesis necessary for the development of the autonomic nervous system, and especially, of respiratory neuronal circuits. In the present study, we examined the role of Phox2b in ventilatory and thermoregulatory responses to hypoxic stress, which are closely related in the postnatal period. Hypoxic stress was generated by strong thermal stimulus, combined or not with reduced inspired O2. To this end, we exposed 6-day-old Phox2b+/− pups and their wild-type littermates (Phox2b+/+) to hypoxia (10% O2) or hypercapnia (8% CO2) under thermoneutral (33°C) or cold (26°C) conditions. We found that Phox2b+/− pups showed less normoxic ventilation (VE) in the cold than Phox2b+/+ pups. Phox2b+/− pups also showed lower oxygen consumption (VO2) in the cold, reflecting reduced thermogenesis and a lower body temperature. Furthermore, while the cold depressed ventilatory responses to hypoxia and hypercapnia in both genotype groups, this effect was less pronounced in Phox2b+/− pups. Finally, because serotonin (5-HT) neurons are pivotal to respiratory and thermoregulatory circuits and depend on Phox2b for their differentiation, we studied 5-HT metabolism using high pressure liquid chromatography, and found that it was altered in Phox2b+/− pups. We conclude that Phox2b haploinsufficiency alters the ability of newborns to cope with metabolic challenges, possibly due to 5-HT signaling impairments.
Keywords: control of breathing, chemosensitivity, hypoxia, hypercapnia, cold
Introduction
The transcription factor Phox2b is a master regulator of the embryonic development of the autonomic nervous system and, especially, of respiratory circuits (Goridis et al., 2010). Phox2b is expressed by afferent pathways from O2-sensitive peripheral chemoreceptors, by chemoresponsive projections of the nucleus tractus solitarius to the ventrolateral medulla, and by central chemoreceptors located in the retrotrapezoid nucleus (RTN; Abbott et al., 2009). In humans, mutations in the Phox2b gene have been determined to cause Congenital Central Hypoventilation Syndrome (CCHS), which is characterized by hypoventilation during sleep and impaired chemosensitivity (Amiel et al., 2009). Knock-in mice harboring Phox2b mutations identified in CCHS patients display apneas and a lack of responsiveness to hypercapnia, and die soon after birth (Dubreuil et al., 2008). These mice show a specific loss of CO2-sensitive cells in the RTN (Dubreuil et al., 2008). Phox2b mutant embryos, in which RTN neurons are specifically depleted, display a reduced phrenic burst frequency and an impaired response to acidification (Dubreuil et al., 2009). Newborn mice carrying one invalidated Phox2b allele (Phox2b+/− mice) show sleep apneas (Durand et al., 2005) and reduced sensitivity to hypercapnia (Dauger et al., 2003; Ramanantsoa et al., 2007). Taken together, these studies demonstrate the important role of Phox2b in the postnatal control of breathing.
In newborns, ventilatory control is tightly coupled with thermoregulation. This coupling allows the fine-tuning of O2 demand and supply, and enables the organism to cope with metabolic challenges (Mortola, 1999; Steiner and Branco, 2002; Chardon et al., 2004; Gargaglioni et al., 2005). In particular, newborns respond to oxygen demand by increasing ventilation, and then, if the demand persists, by decreasing the metabolic rate, thermogenesis and ventilation, an effect designated “hypoxic hypometabolism” (Frappell et al., 1992, 1998; Mortola, 1999, 2005; Bollen et al., 2009). The decrease in thermogenesis reflects a centrally regulated response rather than the mere limitation of oxygen availability (Mortola and Gautier, 1995; Gautier, 1996; Osaka, 2010). Hypoxic hypometabolism is regarded as an important response for the protection of vital organs, especially the brain, from hypoxic stress during the neonatal period. The mechanisms underlying the coupling between ventilatory control and thermoregulation are still elusive (see Osaka, 2010 for a recent review). Our present hypothesis is that Phox2b, which regulates the development of neuronal circuits underlying both functions, may be important to their combined control in newborns.
The aim of the present study was to assess the role of Phox2b in thermoregulation and ventilatory control in newborns. To this end, we measured ventilatory and thermoregulatory responses to hypoxic stress in Phox2b+/− newborn mouse pups. We exposed 6-day-old Phox2b+/− pups and their wild-type littermates (Phox2b+/+) to chemical stimuli (hypoxia or hypercapnia) under cold or thermoneutral conditions. All variables were measured non-invasively in unrestrained pups to avoid any confounding effect of behavioral stress. Finally, since serotonergic neurons depend on Phox2b for their differentiation (Jacob et al., 2007) and are closely involved in respiratory function and in the thermogenic response to cold (Hilaire et al., 2010; Hodges and Richerson, 2010; Madden and Morrison, 2010), we explored the role of serotonin (5-HT) in the above process by measuring brain levels of the neurotransmitter.
Materials and Methods
Animals
The generation and genotyping of Phox2b+/− mice have been described elsewhere (Pattyn et al., 1999). Since respiratory control disorders are frequent in preterm infants and represent a major risk of brain hypoxia, we conducted this study in 6-day-old pups. This postnatal age roughly corresponds to 35 weeks of gestation in humans in terms of central nervous system development (Hagberg et al., 2002). Mice were housed at 21°C with a 12-h light/dark cycle (lights on at 7 a.m.) and fed ad libitum. The animals were randomly assigned to four experimental groups: hypoxia at 26°C, hypoxia at 33°C, hypercapnia at 26°C and hypercapnia at 33°C. Each litter was assigned to one experimental group. All quantifications were performed blind regarding the genotypes, which were determined after all tests were completed. Previous experiments in newborn mice (Bollen et al., 2009) indicated that the physiological responses to cold showed a large interindividual variability, which required a larger sample size in this condition.
Experimental protocols were approved by the Institutional Review Committee. All animal tests were performed in accordance with the European Communities Council Directive (86/809/EEC) regarding the care and use of animals for experimental procedures, in compliance with the regulations of the Ministère de l’Agriculture et de la Forêt, Service Vétérinaire de la Santéet de la Protection Animale (permission # A 94-028-21). All efforts were made to minimize animal suffering, especially by using fully non-invasive functional tests.
Plethysmography
Breathing variables were measured non-invasively using a battery of four whole-body flow barometric plethysmography, allowing the simultaneous measurement of breathing variables as previously described (Bollen et al., 2009). We used whole-body flow barometric plethysmography, rather than head-out plethysmography which provides a relatively direct measure of breathing, to avoid the stress related to restraint. Whole-body flow barometric plethysmography provides semiquantitative measurements of tidal volume and ventilation, and valid measurements of breathing frequency and apnea. Despite these limitations, this latter technique remains the only non-invasive method for studying unrestrained newborn mice.
Each plethysmograph was composed of two 50 ml Plexiglas chambers, immersed in a water-bath to maintain their temperature at 26 or 33°C. A 200 ml min−1 flow of dry air (Brooks airflow stabilizer, Urlo, Holland) was divided into two 100 ml min−1 flows through the chambers. The differential pressure between the chambers (DRUCK–EFFA transducer, Asnières, France) was filtered (bandwidth: 0.15–20 Hz at −3 dB), converted into a digital signal at a sampling rate of 100 Hz, and processed (Labview, National Instruments, Austin, TX, USA). Calibration was done before each session using a built-in pump incorporating a microsyringe (Ito corporation, Fuji, Japan), which injected a sinusoidal airflow with a maximum amplitude of 2 μl and a frequency of 8 Hz into the animal chamber. The limitations of the plethysmographic method in newborn mice (Enhorning et al., 1998; Mortola and Frappell, 1998) have been discussed elsewhere (Lofaso et al., 2007). Because of these limitations, the absolute values given for the tidal volume (VT) and ventilation (VE) are indicative only, whereas the absolute values for breath duration (TTOT) and the duration of apnea, as well as the relative changes from baseline of VT, TTOT, and VE, are reliable.
Breathing variables
TTOT (s), VT (μl g−1 BTPS), and VE (calculated as VT TTOT−1 and expressed in μl s−1 g−1 BTPS) were calculated from apnea-free periods (see apnea determination below). Breath-by-breath values for VE, VT, and TTOT were averaged over consecutive 30 s periods. The baseline levels of these variables were calculated as the mean level during the 3-min of air breathing that preceded exposure to hypoxia or hypercapnia. The time course of VE in response to hypoxia or hypercapnia was expressed as the percentage of change of VE (ΔVE) over consecutive 30 s periods during the 3-min of hypoxia or 6 min of hypercapnia, relative to the last 30 s of air breathing that preceded gaseous exposure. The ventilatory response to hypoxia was also expressed as the percentage change in VE relative to the average baseline VE, using the formula 100·(peak-VE − baseline VE)/baseline VE. The peak-VE response to hypoxia was determined individually as the highest of the 6 mean-VE values (calculated over 30 s) during the 3-min hypoxic exposure. This method took into account possible interindividual differences in the delay to the peak-VE response to hypoxia. We determined the VT and TTOT responses to hypoxia using the same formula, using VT and TTOT values measured at peak-VE. The peak-VE response to hypercapnia was calculated along the same lines. Similarly, HVD was assessed as the maximal decrease of VE in response to hypoxia. We previously reported that VE started to decrease during hypoxia, about 90 s after the switch to hypoxia, but it invariably reached its minimum value over the 3-min period following the switch back to air (Bollen et al., 2009). Although we termed this response HVD, we cannot discard that it involved after effects of hypoxia, i.e., post-hypoxic ventilatory decline (PHVD). HVD and PHVD basically reflect the same neuronal inhibitory mechanisms, the difference being that PHVD is maximally expressed when the excitatory component of the response to hypoxia is no longer present (Coles and Dick, 1996).
Apneas were defined as ventilatory pauses longer than twice the duration of the preceding breath (Sawnani et al., 2004). Total apnea duration was calculated over successive 30 s periods. The apnea response to hypoxia was expressed as the absolute difference between total apnea duration during hypoxia and the same measurement for the 30-s period preceding hypoxia. Percentages were not calculated as some mice had no apneas during normoxia, especially at 26°C.
Heart rate
In a separate experiment, we measured the heart rate (HR) in unrestrained pups. The plethysmographic chambers were equipped with recording platforms composed of four rectangular gold electrodes insulated from one another and embedded in the floor of the chamber, as previously described (Ramanantsoa et al., 2007). Conduction was enhanced using electrode hydrogel (Sekisui Plastics Co., Ltd., Nara, Japan). Signals were digitized at a sampling rate of 1000 Hz (16 bits, PCI-6229, National Instruments, Austin, TX, USA). An ECG signal was obtained when at least three paws were in contact with three different electrodes or, occasionally, when the pup was lying down on the floor. HR was determined from R–R wave peaks after visual selection of continuous ECG segments of 1 s or more with clearly defined QRS waves.
Ultrasonic vocalizations
Ultrasonic vocalizations (USVs) were recorded using an ultrasound bat detector, D230 (Petterson Elektronik AB, Uppsala, Sweden), as previously described (Bollen et al., 2009).
Ventilatory tests
After 15 min of normoxia, hypoxia was achieved by switching the airflow through the plethysmograph to 10% O2 + 90% N2 at the same flow rate (100 ml min−1 per chamber) for 3 min, after which the flow was switched back to normoxia for 12 min (total duration of the session: 30 min). Hypercapnia was achieved by switching the airflow through the plethysmograph to 8% CO2 + 21% O2 + 71% N2 for 6 min, after which the flow was switched back to normoxia for 9 min (total duration of the session: 30 min). We used a longer CO2 stimulus, compared to hypoxia, because the VE response to 8% CO2 reaches its peak-value only after 2–3 min of stimulation in newborn mice (Ramanantsoa et al., 2007).
Thermal challenge
Experiments were conducted at 33 or at 26°C by controlling the temperature of the water surrounding the plethysmographic chambers. Previous analysis in newborn mice has indicated that a temperature of 26°C elicits near-maximal thermogenesis by brown adipose tissue, and may therefore be considered as extreme cold (Bollen et al., 2009). In contrast, 33°C approaches thermoneutrality and corresponds to the temperature within litters of newborn mice in contact with their mother (Renolleau et al., 2001).
Body temperature
We directly measured interscapular skin temperature in all pups in the study, before and immediately after plethysmographic recording, by placing a thermocouple probe at the level of the interscapular region. In a separate experiment, we also measured the emission of infrared (IR) radiation from the skin surface of the interscapular region in the cold, as previously described (Bollen et al., 2009). This was done using an infrared camera (FLIR Systems Thermovision A20, Boston, MA, USA) which was mounted over one of the plethysmograph animal chambers. IR measurement was done through a special window of the plethysmograph chamber made of Zinc Selenide, to ensure permeability to IR emission.
O2 consumption
In a separate experiment, we measured the time course of O2 consumption (corrected for body weight, μl min−1 g−1) in pups exposed to hypoxia (hypercapnia elicits no metabolic response in newborns; Saiki and Mortola, 1996; Putnam et al., 2005) in cold and thermoneutral conditions. To do this, we used a method described previously (Bollen et al., 2009). A fan was placed in the chamber to stir the ambient gas and ensure constant washout kinetics regardless of the position of the pup. This method allowed the calculation of the time course of O2 consumption in conditions of variable FiO2. However the fan disrupted the ventilatory signal, precluding the calculation of the VE/VO2 ratio. We calculated a rough estimation of this ratio within each genotype on subgroups of the hypoxic experiment weight-matched to the VO2 experiment groups. To do this, we divided individual VE values by the value of VO2 of the corresponding genotype group over the same periods (3-min preceding hypoxia, time of the peak-VE response to hypoxia, and time of the minimum VE response to hypoxia).
Analysis of serotonergic metabolism
Brains were dissected and kept at −80°C until analysis as previously reported (Ramirez and Viemari, 2005). Brain levels of serotonin (5-HT), its precursor l-tryptophan (l-Trp) and its main 5-HT metabolite from the monoamine oxidase A (MAOA) degradation pathway, 5-hydroxyindoleacetic acid (5-HIAA), were measured using high pressure liquid chromatography (HPLC) and electrochemical detection (Waters System: pump P510, electrochemical detector EC2465; Atlantis column DC18; mobile phase: citric acid, 50 mM; orthophosphoric acid, 60 mM; sodium octanesulfonic acid, 0.112 mM; EDTA: 0.06 mM, methanol; pH 3.01).
Statistics
All respiratory variables were averaged over consecutive 30 s periods and subjected to analysis of variance with genotype (Phox2b+/− versus wild-type Phox2b+/+) and temperature (26 versus 33°C) as the between-subjects factors. Litter had no significant effect, neither as a main between-factor nor in interaction with other factors analyzed, and will not be mentioned further. We also conducted complementary analyses using weight-matched groups to determine whether any differences between Phox2b+/+ and Phox2b+/− pups were related to weight. Since all analyses yielded the same statistical results as those obtained using the entire population, this variable will not be mentioned further. In all tests, a p-value of ≤0.05 was considered to indicate a significant difference. Group means compared by ANOVA are presented along with SD in the text and the tables. SEM are used in the figures for the sake of clarity. All statistical analyses were conducted using StatView 5 (Abacus Concepts, Berkeley, CA, USA).
Results
Greater temperature drop in the cold in Phox2b+/− pups
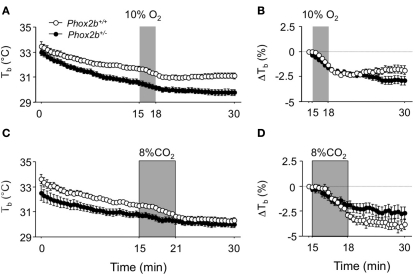
Phox2b+/− pups weighed significantly less than their wild-type littermates (Table 1). Before plethysmography, body temperatures measured (with a thermocouple) immediately after taking the pup from the litter were highly similar in Phox2b+/− and Phox2b+/+ pups (Table 1). In contrast, when exposed to 26°C, body temperatures (measure by infrared thermography) were lower in Phox2b+/− pups than in their Phox2b+/+littermates, in both hypoxic and hypercapnic studies (Figure 1). Thus, Phox2b+/− pups showed a reduced ability to maintain body temperature in the cold.
Table 1.
Weight and temperature of 6-day-old Phox2b+/− and Phox2b+/+ mice.
| 33°C | 26°C | G × T | |||||
|---|---|---|---|---|---|---|---|
| Phox2b+/+ | Phox2b+/− | p | Phox2b+/+ | Phox2b+/− | p | p | |
| HYPOXIA STUDY | |||||||
| n | 41 | 43 | 84 | 85 | |||
| Weight (g) | 3.4 (0.5) | 2.8 (0.4) | <0.0001 | 3.7 (0.6)* | 3.0 (0.5)* | <0.0001 | NS |
| T° initial (°C) | 33.9 (1.1) | 33.7 (1.0) | NS | 33.5 (1.5) | 33.3 (1.4) | NS | NS |
| T° final (°C) | 34.8 (0.6) | 34.6 (0.7) | NS | 30.5 (1.1)**** | 29.8 (1.4)**** | <0.0003 | <0.0608 |
| T° chamber (°C) | 33.0 (0.3) | 33.0 (0.3) | NS | 26.0 (0.3) | 26.0 (0.2) | NS | NS |
| T° infrared (°C) | – | – | 31.8 (0.6) | 30.8 (1.1) | 0.0001 | – | |
| HYPERCAPNIA STUDY | |||||||
| n | 34 | 36 | 51 | 45 | |||
| Weight (g) | 3.7 (0.5) | 3.4 (0.7) | <0.0439 | 3.7 (0.5) | 3.3 (0.7) | 0.0006 | NS |
| T° initial (°C) | 34.3 (1.1) | 34.4 (1.2) | NS | 34.1 (1.4) | 33.7 (1.4)* | NS | NS |
| T° final (°C) | 34.8 (1.2) | 34.9 (0.8) | NS | 30.8 (1.0)**** | 30.4 (0.8)**** | 0.0321 | NS |
| T° chamber (°C) | 33.0 (0.4) | 32.9 (0.4) | NS | 26.2 (0.4) | 26.2 (0.4) | NS | |
Weight and interscapular skin temperature were measured immediately after taking the pup from the litter (T° initial). The p-value refers to the comparison between Phox2b+/+ and Phox2b+/− groups. Comparison of 33 versus 26°C for each genotype group; *p < 0.05; ****p < 0.0001. Values indicate mean (SD) for each group. G × T: p-value for Genotype by Temperature interaction. Note that G × T interactions were marginally significant in the hypoxic study and therefore that genotype-related differences in final temperatures are unwarranted. Infrared temperature were not measured at 33°C, due to the lack of infrared image of the pup in thermoneutral conditions.
Figure 1.
Body temperature (Tb) in Phox2b+/+ and Phox2b+/− pups exposed to hypoxia [(A,B) 10% O2, shaded, 20 Phox2b+/+, and 31 Phox2b+/− pups] or hypercapnia [(C,D) 8% CO2, shaded, 12 Phox2b+/+, and 20 Phox2b+/− pups] at an ambient temperature of 26°C. Body temperature was measured at the level of interscapular brown adipose tissue by infrared thermography. Values are absolute levels (A,C) and relative changes from pre-stimulus levels (B,D). Body temperature progressively decreased, revealing an inability to maintain core temperature, especially in Phox2b+/− pups (A,C). Both hypoxic and hypercapnic stimuli induced a reduction in body temperature (A,C). Hypoxia induced similar temperature losses in Phox2b+/+ and Phox2b+/− pups, but temperature recovery long after the return to normoxia was blunted only in Phox2b+/− pups (B). In contrast, the temperature loss after hypercapnia was blunted in Phox2b+/− pups (D). Values indicate means ± SEM for the group.
Depressed baseline ventilation in the cold in Phox2b+/− pups
At 33°C, baseline normoxic VE values were highly similar in Phox2b+/+ and Phox2b+/− pups (Table 2). The cold stimulated VE in both genotype groups, although this effect was significantly lower in Phox2b+/− pups (Table 2). This difference was further supported by apnea analysis. Apnea duration was longer in Phox2b+/− than in Phox2b+/+ pups both at 33 and at 26°C (Table 2). Whereas the effect of the cold on apnea duration in Phox2b+/+ pups was minimal, it significantly increased apnea duration in Phox2b+/− pups (Table 2). Thus, the ventilatory response to cold was markedly disrupted in Phox2b+/− pups. The HR was diminished by the cold in both groups, without significant genotype-related differences. Behaviorally, the cold similarly stimulated movement and USV generation in Phox2b+/− and Phox2b+/+ pups (Table 2), suggesting that the perception of cold was not altered in Phox2b+/− pups.
Table 2.
Baseline cardiorespiratory variables.
| 33°C | 26°C | G × T | |||||
|---|---|---|---|---|---|---|---|
| Phox2b+/+ | Phox2b+/− | p | Phox2b+/+ | Phox2b+/− | p | p | |
| n | 75 | 79 | 135 | 130 | |||
| VE (μl s−1 g−1) | 16.3 (2.3) | 16.3 (2.5) | NS | 29.8 (10.8)**** | 21.5 (11.7)**** | <0.0001 | <0.0001 |
| VT (μl g−1) | 5.4 (0.6) | 5.6 (0.8) | NS | 8.6 (1.70)**** | 7.9 (2.8)**** | 0.0098 | <0.0148 |
| TTOT (s) | 0.34 (0.1) | 0.35 (0.1) | NS | 0.3 (0.1) | 0.4 (0.1)**** | <0.0001 | <0.0001 |
| Apneas (s) | 0.3 (0.3) | 0.7 (1.1) | 0.0024 | 0.2 (1.2) | 2.3 (4.4)*** | <0.0001 | <0.0011 |
| MVT (s) | 2.0 (2.8) | 1.91 (2.9) | NS | 2.6 (3.5) | 3.4 (4.3)** | NS | NS |
| USV (s) | 0.02 (0.07) | 0.02 (0.04) | NS | 0.51 (0.57)**** | 0.48 (0.65)**** | NS | NS |
| VO2 (ml/min/g) n | 43.9 (13.3) 8 | 38.6 (7.1) 5 | NS | 71.8 (5.8)**** 10 | 52.4 (19.2) 17 | <0.0049 | NS† |
| HR (bpm) n | 511 (68) 27 | 492 (64) 22 | NS | 461 (60)*** 57 | 453 (70)* 61 | NS | NS |
Values indicate mean (SD) for each group, calculated over the 3-min preceding the hypoxic or hypercapnic stimulus. Apnea, MVT, USV = total apnea, movement duration, and ultrasonic vocalization(s) over 30 s periods, respectively. HR: heart rate (beats min−1). Comparison of 33 versus 26°C for each genotype group; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001; p-value refers to the comparison between Phox2b+/+ and Phox2b+/− groups. G × T: p-value for Genotype by Temperature interaction. †!:complete analysis of VO2 over the entire air period yielded significant interaction (see text). Oxygen consumption (VO2) and heart rate (HR) studies were conducted on separate groups of mice. The estimated VE/VO2 ratios on weighted matched subgroups indicated a significant difference between Phox2b+/+ and Phox2b+/− groups at 33°C [20.78 (1.92), n = 11, versus 38.77 (3.88), n = 22, p < 0.0001], but not at 26°C [29.31 (8.29), n = 44 versus 27.37 (10.21), n = 56, NS].
Abnormal ventilatory response to hypoxia in the cold in Phox2b+/− pups
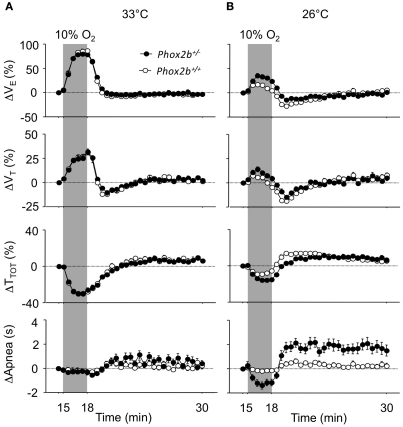
At 33°C, the ventilatory response to hypoxia displayed similar trends in the Phox2b+/+ and Phox2b+/− groups (Figure 2A), despite small but significant differences in the peak hyperpneic VE response and in the HVD (Table 3). In both genotype groups, the cold depressed the VE response to hypoxia compared to 33°C conditions, leading to reduced hyperpneic responses and an increased HVD (Figure 2B; Table 3). However, these effects of the cold were less marked in Phox2b+/− than in Phox2b+/+ pups (Figures 2A,B; Table 3). As a result, in the cold, Phox2b+/− pups showed a larger hyperpneic response to hypoxia than Phox2b+/+ pups, due mostly to the greater drop in TTOT (Figure 2B; Table 3). In line with their stronger hyperpneic response, the HVD in Phox2b+/− pups was depressed when compared to Phox2b+/+ pups (Figure 2B; Table 3). Estimated VE/VO2 ratios at the time of the peak-VE response to hypoxia were larger in Phox2b+/− pups, compared to Phox2b+/+ pups at both temperatures (33°C: 214 ± 22 versus 85 ± 11, p < 0.0001; 26°C: 52 ± 24 versus 38 ± 10, p < 0.0001). In contrast, estimated VE/VO2 ratios were smaller in Phox2b+/− pups, compared to Phox2b+/+ during HVD (33°C: 15 ± 1 versus 17 ± 1, p < 0.0006; 26°C: 22 ± 9 versus 32 ± 10, p < 0.0001).
Figure 2.
Ventilatory responses to hypoxia (10% O2, shaded) in Phox2b+/+ and Phox2b+/− pups at 33°C (A) and 26°C (B). VE: ventilation; TTOT: breath duration; VT: tidal volume. Apnea: total apnea duration/30 s. Values are expressed as percentage changes from pre-hypoxic levels for VE, VT, and TTOT. Apnea durations are expressed as absolute changes from pre-hypoxic levels. Latency to peak-VE from the onset of hypoxia shows wide interindividual variability (see Table 3), which smoothens the difference between the mean-VE curves. At 33°C, breathing variables are similar in Phox2b+/+ and Phox2b+/− pups, although Phox2b+/− pups have a slightly smaller VE response to hypoxia (see Table 3 for statistical analyses). The HVD is more sustained in Phox2b+/− pups than in Phox2b+/+ pups exposed to cold. Values indicate means ± SEM for each group. See Table 1 for group sizes.
Table 3.
Ventilatory responses to hypoxia and hypercapnia.
| 33°C | 26°C | G × T | |||||
|---|---|---|---|---|---|---|---|
| Phox2b+/+ | Phox2b+/− | p | Phox2b+/+ | Phox2b+/− | p | p | |
| PEAK-VE RESPONSE TO HYPOXIA | |||||||
| n | 41 | 43 | 84 | 85 | |||
| ΔVE (%) | 95 (22) | 80 (17) | 0.0011 | 29 (19)**** | 51 (33)**** | <0.0001 | <0.0001 |
| ΔVT (%) | 32 (14) | 27 (12) | NS | 13 (13)**** | 23 (21) | 0.0004 | <0.0009 |
| ΔTTOT (%) | −32 (7) | −29 (8) | NS | −12 (8)**** | −18 (10)**** | 0.0001 | <0.0003 |
| Peak-VE latency (s) | 143 (29) | 148 (30) | NS | 99 (41)**** | 110 (44)**** | NS | NS |
| HYPOXIC VENTILATORY DECLINE | |||||||
| n | 41 | 43 | 84 | 85 | |||
| ΔVE (%) | −11 (7) | −7 (9) | 0.0173 | −33 (12)**** | −25 (18)**** | 0.0007 | NS |
| ΔVT (%) | −12 (10) | −8 (9) | NS | −23 (10)**** | −17 (13)*** | 0.0021 | NS |
| ΔTTOT (%) | 0 (14) | −1 (14) | NS | 18 (20) | 13 (17) | NS | NS |
| PEAK-VE RESPONSE TO HYPERCAPNIA | |||||||
| n | 34 | 36 | 51 | 45 | |||
| ΔVE (%) | 150 (62) | 118 (70) | 0.0489 | 96 (47)**** | 121 (71) | 0.0436 | <0.0044 |
| ΔVT (%) | 98 (23) | 83 (30) | 0.0300 | 81 (30)** | 89 (44) | NS | <0.0314 |
| ΔTTOT (%) | −19 (12) | −13 (13) | 0.0346 | −4 (14)**** | −11 (18) | 0.0440 | 0.0050 |
| Peak-VE latency (s) | 294 (65) | 292 (59) | NS | 194 (71)**** | 250 (90)* | 0.0010 | 0.0129 |
Values are percentage changes from pre-stimulus values. The peak-VE response to hypoxia and hypoxic ventilatory decline were calculated individually over the entire 3 min of hypoxia and the 3-min post-hypoxic period, respectively. The peak-VE response to hypercapnia was calculated over the entire 6 min of hypercapnia. Values indicate mean (SD) for each group. The p-value refers to the comparison between Phox2b+/+ and Phox2b+/ groups. Comparison of 33 versus 26°C for each genotype group; *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. G × T: p-value for Genotype by Temperature interaction. Note that the G × T interaction was marginally significant for VT.
Apneas were nearly absent and showed no significant changes in response to hypoxia in Phox2b+/+ pups at either temperature. In contrast, apneas occurred in Phox2b+/− pups, and showed longer total durations than in Phox2b+/+ pups, especially in the cold; apneas markedly decreased in response to hypoxia, and then markedly increased during the HVD (Figure 2B). However, total apnea duration remained low in Phox2b+/− pups (<3 s out of 30 s; Figure 2B).
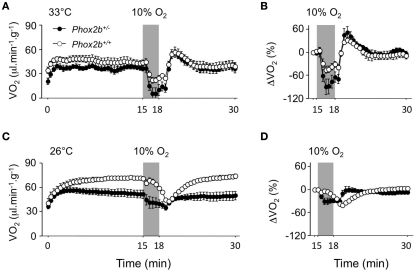
Disrupted VO2 response to cold in Phox2b+/− pups
Firstly, due to their complex pattern, VO2 data were analyzed over the entire normoxic periods in cold and thermoneutral conditions (Figure 3). The comparison of the time courses of VO2 between Phox2b+/− and Phox2b+/+ pups yielded different results at 33 and 26°C (Genotype by Temperature by Time interaction, p < 0.0001). At 33°C, the time courses of VO2 were similar in Phox2b+/− and Phox2b+/+ pups (Genotype by Time interaction: NS; Figure 3A). In contrast, at 26°C, these time courses were markedly different in Phox2b+/− and Phox2b+/+ pups, due to smaller VO2 increase and steady state in Phox2b+/− pups (Genotype by Time interaction, p < 0.0001; Figure 3C; Table 2). Thus, the VO2 response to cold was markedly altered in Phox2b+/− pups. The reduced VO2 response to cold in Phox2b+/− pups is in line with their lower body temperatures and VE (Table 2).
Figure 3.
Oxygen consumption (VO2) in Phox2b+/+ and Phox2b+/− pups exposed to hypoxia (10% O2, shaded) at 33°C [(A,B) Phox2b+/+: n = 8 and Phox2b+/−: n = 5] and at 26°C [(C,D) Phox2b+/+: n = 10 and Phox2b+/−: n = 17). Values are absolute levels (A,C) and relative changes from pre-hypoxic levels (B,D). Regardless of temperature, hypoxia depresses VO2 in both genotype groups. At 33°C, the drop in VO2 is larger in Phox2b+/− pups. At 26°C, the VO2 response to hypoxia is markedly altered in Phox2b+/− compared to Phox2b+/+ pups. Values indicate means ± SEM for each group.
Disrupted hypoxic hypometabolism in Phox2b+/− pups
The analysis of VO2 response to hypoxia (especially when expressed as percent changes from pre-hypoxic levels) yielded different results in Phox2b+/− and Phox2b+/+ pups (Genotype by Temperature by Time interaction, p < 0.0001; Figures 3B,D). At 33°C, the magnitude of the drop in VO2 (relative to pre-hypoxic levels) was greater in Phox2b+/− pups than in wild-type controls although this difference was not significant (Genotype by Time interaction: NS; Figure 3B). In contrast, at 26°C, the entire pattern of VO2 changes was altered in Phox2b+/− pups, so that the time course of VO2, relative to pre-hypoxic levels, was practically in phase opposition between Phox2b+/− and Phox2b+/+ pups (Genotype by Time interaction: p < 0.0001; Figure 3D). These results indicate that the metabolic response to the cold and hypoxia was profoundly affected by the Phox2b mutation.
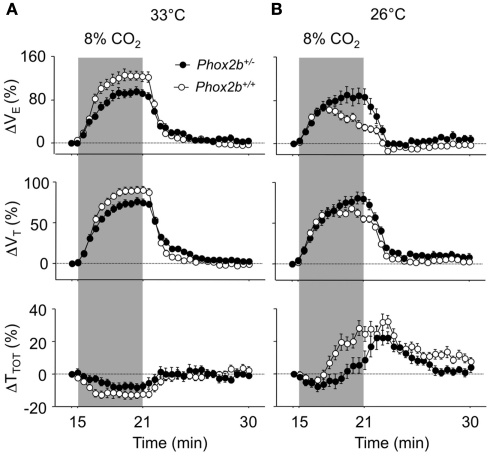
Abnormal ventilatory response to hypercapnia in the cold in Phox2b+/− pups
At 33°C, the VE response to hypercapnia was significantly reduced in Phox2b+/− than in Phox2b+/+ pups, as previously reported (Dauger et al., 2003; Durand et al., 2005; Ramanantsoa et al., 2007). This difference in VE was due to lower responses in both TTOT and VT, but mostly VT, in Phox2b+/− pups (Figures 4A,B; Table 3).
Figure 4.
Ventilatory responses to hypercapnia (8% CO2, shaded) in Phox2b+/+ and Phox2b+/− pups at 33°C (A) and 26°C (B). See legend of Figure 2 for abbreviations. At 33°C, the VE response to hypercapnia was significantly lower in Phox2b+/− pups compared to Phox2b+/+ pups. This difference was reversed at 26°C, due to the lack of a hypercapnic ventilatory decline in Phox2b+/− pups. Values indicate means ± SEM for each group. See Table 1 for group sizes.
The cold depressed the ventilatory response to hypercapnia in Phox2b+/+ but not in Phox2b+/− pups (Figures 4A,B; Table 3). This difference was mainly due to the fact that, in the cold, VE was reduced in Phox2b+/+ pups before the end of exposure to hypercapnia (hypercapnic ventilatory decline). In contrast, in Phox2b+/− pups, the VE response to hypercapnia was sustained throughout the hypercapnic stimulus (Figures 4A,B; Table 3). The difference in the VE response to hypercapnia between the two genotype groups was mainly due to differences in TTOT. The absence of a hypercapnic ventilatory decline in Phox2b+/− pups led to a larger VE response during the last 3 min of hypercapnic exposure in Phox2b+/− than in Phox2b+/+ pups (Figures 4A,B; Table 3).
These differences in the VE response to CO2 were apparently not related to arousal. In fact, at 33°C, movement duration (MVT) and USV duration in response to hypercapnia were practically absent in both genotype groups. In the cold, the USV and MVT responses to hypercapnia were similar in both genotype groups (data not shown).
Impaired brain 5-HT metabolism in Phox2b+/− pups
We found highly similar 5-HT levels in the two groups, although concentrations of l-Trp (a 5-HT precursor) and 5-HIAA (a 5-HT catabolite) were higher in Phox2b+/− preparations (Table 4). The 5-HT/5-HIAA ratio, a marker of 5-HT turnover rate, was significantly lower in Phox2b+/− preparations (Table 4). On the other hand, concentrations of adrenaline, dopamine and the metabolite DOPAC were very similar in Phox2b+/− and Phox2b+/+ pups (data not shown).
Table 4.
Detection of serotonin in the brains of 6-day-old Phox2b+/+ (n = 20) and Phox2b+/− (n = 13) pups.
| Phox2b+/+ | Phox2b+/− | p-Value | |
|---|---|---|---|
| 5-HT | 5.73 (1.45) | 6.41 (1.49) | 0.0954 |
| l-Trp | 62.65 (21.96) | 82.99 (32.48) | 0.0196 |
| 5-HIAA | 2.47 (0.72) | 3.48 (1.14) | 0.0019 |
| 5-HT/5-HIAA | 2.48 (0.84) | 1.96 (0.54) | 0.0288 |
Serotonin (5-HT), the serotonin precursor l-tryptophan (l-Trp), and its degradation product 5-hydroxyindoleacetic acid (5-HIAA) were quantified using HPLC. Data indicate abnormal 5-HT metabolism in Phox2b+/− pups. Contents are expressed in nanogram per gram of wet brain tissue. Values indicate mean (SD) for each group.
Discussion
The present results confirm and extend our previous findings regarding the impairment of respiratory control in Phox2b+/− pups (Dauger et al., 2003; Ramanantsoa et al., 2006, 2007). Under thermoneutral conditions (33°C), Phox2b+/− pups show nearly normal baseline breathing patterns and a reduced ventilatory response to hypercapnia, and to a lesser extent, to hypoxia. More importantly, the present study reveals important impairments of the ventilatory and thermoregulatory responses to cold. In the cold, Phox2b+/− pups show reduced normoxic ventilation and oxygen consumption (VO2) and a lower body temperature when compared to Phox2b+/+ pups. Furthermore, the inhibitory effects of the cold on the ventilatory responses to hypoxia and hypercapnia are blunted in Phox2b+/− pups. Finally, these physiological impairments are associated with a modified turnover of brain 5-HT.
Impaired thermogenic responses to cold
The blunted VO2 and temperature responses to cold in Phox2b+/− pups are novel findings that suggest that the phenotypic defects caused by Phox2b haploinsufficiency are more important than previously reported. In both genotype groups, body temperature progressively decreased in spite of constant high VO2 and VE levels during exposure to cold. The increase in VO2 is due to the increase in oxidative metabolism caused by heat production by brown adipose tissue (Darnall et al., 2006). The thermogenic response to cold is a critical defensive response, present at birth (Frappell et al., 1998; Malik and Fewell, 2003) in numerous species (Maskrey, 1990; Cameron et al., 2000; Mortola, 2005) including mice (Bollen et al., 2009). Thus, Phox2b haploinsufficiency, which has a limited physiological impact under normal temperature conditions, may be of greater important under cold conditions. It is unclear whether these impairments are related to heat dissipation, which depends on the central control of vasomotricity and may rely on Phox2b-dependent structures (Pattyn et al., 1999).
Chemosensitivity disorders under thermoneutral conditions
Ventilatory results obtained in Phox2b+/− pups under thermoneutral conditions confirm previous observations carried out at similar ages (Dauger et al., 2003; Durand et al., 2005; Ramanantsoa et al., 2007). In air, VE levels at 33°C were similar in Phox2b+/+ and Phox2b+/− pups; however, the VE response to hypercapnia was blunted in Phox2b+/− pups, an effect previously shown to be present on postnatal day 2 (P2), but recovered by around P10 (Dauger et al., 2003). In contrast, the slightly diminished responses to hypoxia under thermoneutral conditions, both in terms of the initial hyperpneic response and the HVD, are novel findings that contrast with the stronger HVD observed on P2 (Dauger et al., 2003). This difference is possibly due to the fact that the resetting of peripheral chemoreceptors occurs between 6 and 12 h postnatally in mice (Renolleau et al., 2001). Therefore, the hyperpneic response to hypoxia is very immature in 2-day-old pups, possibly masking the effects of the Phox2b mutation. The impairment of the hypoxic ventilatory response could thus be related to the previously described developmental defect in petrosal chemosensory neurons (Dauger et al., 2003).
Ventilatory and metabolic responses to hypoxia in the cold
In both genotype groups, the VE response to hypoxia was dramatically decreased by the cold, leading to a smaller initial hyperpneic component and to larger ventilatory decline, as previously reported (Bollen et al., 2009). In fact, the need for increased thermogenesis in the context of reduced oxygen availability induces a shift toward metabolic depression, rather than increased ventilation (Mortola, 2005). This depressive effect of the cold was reduced in Phox2b+/− pups, as shown by their larger hyperpneic responses and smaller HVD when compared to Phox2b+/+ pups.
As a rule, numerous factors contribute to the HVD: decreased inputs from the carotid bodies, central inhibitory neurotransmitters, reduced PaCO2, decreased metabolism (Waters and Gozal, 2003). Among these, a defective metabolic response to hypoxia could account for the blunted HVD in Phox2b+/− pups. Not only was the overall VO2 response to the cold blunted in Phox2b+/− pups, the decrease in VO2 caused by hypoxia presented major time-dependent differences between Phox2b+/+and Phox2b+/− pups at both temperatures. Under thermoneutral conditions, the drop in VO2 was greater in Phox2b+/− than in Phox2b+/+ pups. In the cold, the time course of VO2 in Phox2b+/− and Phox2b+/+ pups was practically in opposite-phase, reflecting metabolic responses that were qualitatively and quantitatively different in these two groups. Although the differences in VO2 probably affected the ventilatory response to hypoxia in the cold, it is unlikely that they fully accounted for these genotype-related differences, a conclusion supported by the respective time courses of VO2 and VE (Figures 2 and 3). Although technical limitations precluded the simultaneous measurement of VE and VO2, the qualitative differences between the VE and VO2 responses to hypoxia in the cold and their estimated ratios suggest that the coupling between these two responses is markedly affected in Phox2b+/− pups.
Impaired ventilatory responses to hypercapnia
The present study confirms that the ventilatory response to hypercapnia in Phox2b+/− pups is diminished, as previously reported (Dauger et al., 2003; Ramanantsoa et al., 2007). In Phox2b+/+ pups only, the hyperpneic response to hypercapnia was depressed in the cold, as compared to thermoneutral conditions, in line with previous results in 2-day-old mice (Ramanantsoa et al., 2007) and 4- to 6-day-old rats (Saiki and Mortola, 1996). The main consequence of the depressive effect of the cold on the VE response to hypercapnia was that the VE of Phox2b+/+ pups decreased after 3 min of hypercapnia, an effect that was not observed in Phox2b+/− pups. Previous studies have reported that the expiratory duration increases slightly during sustained hypercapnia in 5-day-old rats under thermoneutral conditions, an effect ascribed to GABAergic mechanisms (Abu-Shaweesh et al., 1999). The mechanisms underlying the depressive effects of the cold on the ventilatory response to hypercapnia may be related to the fact that an increase in VE would promote heat dissipation and a reduction in body temperature (Mortola, 2005). Thus, the abnormal metabolic response of Phox2b+/− pups in response to cold may at least partly account for their altered response to hypercapnia when compared to Phox2b+/+ pups.
The role of 5-HT metabolism
Chromatographic analysis indicates that 5-HT metabolism is impaired in the brain of Phox2b+/− pups. Whereas 5-HT levels were similar in Phox2b+/− and Phox2b+/+ pups, the concentrations of its precursor and catabolite were higher, and the 5-HT turnover rate lower, in Phox2b+/− preparations. The abnormal 5-HT metabolism revealed by these results might be ascribable to the impaired development of 5-HT neurons, since Phox2b is closely involved in the production of essential enzymes for catecholamine biosynthesis and the selection between a motoneuronal and a 5-HT neuronal fate in the embryonic central nervous system (Pattyn et al., 2003; Alenina et al., 2006; Coppola et al., 2010). However, it is unclear generation of 5-HT neurons was disrupted in circuits involved in thermoregulation and respiratory control. Because the present chromatographic data were collected in the whole brain, it is difficult to specifically associate 5-HT metabolism disorders and physiological disorders in Phox2b+/− pups.
However, the putative association between 5-HT metabolism and early respiratory control disorders in Phox2b+/− pups is in line with previous studies in newborn mice with a genetic impairment in 5-HT signaling. In particular, newborn transgenic mice lacking serotonergic neurons display severe apnea and mortality (Hodges et al., 2009). Transgenic mice lacking central 5-HT neurons (Lmx1bf/f/p mice) show a 50% reduction in the hypercapnic ventilatory response and insufficient heat production under cold conditions (Hodges and Richerson, 2008; Hodges et al., 2008). Adult Pet-1 null mice, which retain 30% of their central 5-HT neurons, show gender-related impairments in baseline ventilation, hypercapnic ventilatory responses, and heat production in response to cold (Hodges et al., 2011). Taken together, these studies strongly support the idea that 5-HT neurons play an integrative role in the control of ventilation in response to metabolic and thermoregulatory demands, as previously proposed (reviewed in Hodges et al., 2011). Therefore, the impairment of 5-HT metabolism in Phox2b+/− pups may account for their reduced ventilatory drive and disrupted hypoxic hypometabolism response to cold.
Clinical implications
The present study suggests that Phox2b haploinsufficiency compromises the newborn’s ability to cope with hypoxia and cold. Hypoxia is the most common cause of brain damage in newborns, and hypothermia is also associated with increased morbidity and mortality, although moderate hypothermia may be neuroprotective in infants with hypoxic-ischemic encephalopathy (Johnston et al., 2011). To date, the only known link between Phox2b mutations and physiological disorders concerns CCHS. However, the disease-causing mechanism of CCHS is not Phox2b haploinsufficiency (i.e., the putative consequence of the invalidation of one allele of Phox2b) but rather a toxic gain of function or a dominant-negative effect of a Phox2b polyalanine expansion (Amiel et al., 2009), which causes a much more severe phenotype than Phox2b haploinsufficiency. The analysis of the parents and family members of CCHS patients (5–10% of CCHS mutations are inherited) has revealed milder forms of CCHS than previously identified, generally due to shorter polyalanine expansions or non-polyalanine PHOX2B mutations (Weese-Mayer et al., 2010). These subclinical phenotypes suggest that PHOX2B mutations may induce more frequent and less severe autonomic dysregulations than those observed in CCHS. In this respect, our present results indicate that Phox2b mutations with mild consequences under normal conditions could nevertheless profoundly alter the physiological response to extreme conditions.
Conclusion
In the present study, we show that the cold markedly alters the respiratory phenotype of Phox2b+/− pups, as reflected by their impaired baseline ventilation and ventilatory responses to hypercapnia and hypoxia. The ventilatory impairments seen in Phox2b+/− pups were at least partially accounted for by their reduced ability to induce thermogenic responses to cold. Thus Phox2b haploinsufficiency, which has mild effects on vital functions under standard conditions, may in fact profoundly alter the ability to cope with severe metabolic challenges during early postnatal development.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are greatly indebted to Dr. Jean-François Brunet and Dr. Christo Goridis for extensive advice and valuable discussions throughout the project and to Thomas Bourgeois for his contribution to experimental work. Grants: This study was supported by the Institute National de la Santé et de la Recherche Médicale (INSERM), Society de Pneumologie de Langue Française (SPLF), Association Française du Syndrome d’Ondine (AFSO), and Centre National de la Recherche Scientifique (CNRS).
References
- Abbott S. B., Stornetta R. L., Fortuna M. G., Depuy S. D., West G. H., Harris T. E., Guyenet P. G. (2009). Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci. 29, 5806–5819 10.1523/JNEUROSCI.1106-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Shaweesh J. M., Dreshaj I. A., Thomas A. J., Haxhiu M. A., Strohl K. P., Martin R. J. (1999). Changes in respiratory timing induced by hypercapnia in maturing rats. J. Appl. Physiol. 87, 484–490 [DOI] [PubMed] [Google Scholar]
- Alenina N., Bashammakh S., Bader M. (2006). Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2, 5–10 10.1007/s12015-006-0002-2 [DOI] [PubMed] [Google Scholar]
- Amiel J., Dubreuil V., Ramanantsoa N., Fortin G., Gallego J., Brunet J. F., Goridis C. (2009). PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir. Physiol. Neurobiol. 168, 125–132 10.1016/j.resp.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Bollen B., Bouslama M., Matrot B., Rotrou Y., Vardon G., Lofaso F., Van den Bergh O., D’Hooge R., Gallego J. (2009). Cold stimulates the behavioral response to hypoxia in newborn mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1503–R1511 10.1152/ajpregu.90582.2008 [DOI] [PubMed] [Google Scholar]
- Cameron Y. L., Merazzi D., Mortola J. P. (2000). Variability of the breathing pattern in newborn rats: effects of ambient temperature in normoxia or hypoxia. Pediatr. Res. 47, 813–818 10.1203/00006450-200006000-00022 [DOI] [PubMed] [Google Scholar]
- Chardon K., Bach V., Telliez F., Cardot V., Tourneux P., Leke A., Libert J. P. (2004). Effect of caffeine on peripheral chemoreceptor activity in premature neonates: interaction with sleep stages. J. Appl. Physiol. 96, 2161–2166 10.1152/japplphysiol.01160.2003 [DOI] [PubMed] [Google Scholar]
- Coles S. K., Dick T. E. (1996). Neurones in the ventrolateral pons are required for post-hypoxic frequency decline in rats. J. Physiol. 497(Pt 1), 79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola E., d’Autreaux F., Rijli F. M., Brunet J. F. (2010). Ongoing roles of Phox2 homeodomain transcription factors during neuronal differentiation. Development 137, 4211–4220 10.1242/dev.056747 [DOI] [PubMed] [Google Scholar]
- Darnall R. A., Ariagno R. L., Kinney H. C. (2006). The late preterm infant and the control of breathing, sleep, and brainstem development: a review. Clin. Perinatol. 33, 883–914; abstract x. 10.1016/j.clp.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Dauger S., Pattyn A., Lofaso F., Gaultier C., Goridis C., Gallego J., Brunet J. F. (2003). Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 130, 6635–6642 10.1242/dev.00866 [DOI] [PubMed] [Google Scholar]
- Dubreuil V., Ramanantsoa N., Trochet D., Vaubourg V., Amiel J., Gallego J., Brunet J. F., Goridis C. (2008). A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc. Natl. Acad. Sci. U.S.A. 105, 1067–1072 10.1073/pnas.0709115105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil V., Thoby-Brisson M., Rallu M., Persson K., Pattyn A., Birchmeier C., Brunet J. F., Fortin G., Goridis C. (2009). Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J. Neurosci. 29, 14836–14846 10.1523/JNEUROSCI.2623-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand E., Dauger S., Pattyn A., Gaultier C., Goridis C., Gallego J. (2005). Sleep-disordered breathing in newborn mice heterozygous for the transcription factor Phox2b. Am. J. Respir. Crit. Care Med. 172, 238–243 10.1164/rccm.200411-1528OC [DOI] [PubMed] [Google Scholar]
- Enhorning G., van Schaik S., Lundgren C., Vargas I. (1998). Whole-body plethysmography, does it measure tidal volume of small animals? Can. J. Physiol. Pharmacol. 76, 945–951 10.1139/y99-002 [DOI] [PubMed] [Google Scholar]
- Frappell P. B., Dotta A., Mortola J. P. (1992). Metabolism during normoxia, hyperoxia, and recovery in newborn rats. Can. J. Physiol. Pharmacol. 70, 408–411 10.1139/y92-051 [DOI] [PubMed] [Google Scholar]
- Frappell P. B., Leon-Velarde F., Aguero L., Mortola J. P. (1998). Response to cooling temperature in infants born at an altitude of 4,330 meters. Am. J. Respir. Crit. Care Med. 158, 1751–1756 [DOI] [PubMed] [Google Scholar]
- Gargaglioni L. H., Steiner A. A., Branco L. G. (2005). Involvement of serotoninergic receptors in the anteroventral preoptic region on hypoxia-induced hypothermia. Brain Res. 1044, 16–24 10.1016/j.brainres.2005.02.069 [DOI] [PubMed] [Google Scholar]
- Gautier H. (1996). Interactions among metabolic rate, hypoxia, and control of breathing. J. Appl. Physiol. 81, 521–527 [DOI] [PubMed] [Google Scholar]
- Goridis C., Dubreuil V., Thoby-Brisson M., Fortin G., Brunet J. F. (2010). Phox2b, congenital central hypoventilation syndrome and the control of respiration. Semin. Cell Dev. Biol. 21, 814–822 10.1016/j.semcdb.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Hagberg H., Peebles D., Mallard C. (2002). Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment. Retard Dev. Disabil. Res. Rev. 8, 30–38 10.1002/mrdd.10007 [DOI] [PubMed] [Google Scholar]
- Hilaire G., Voituron N., Menuet C., Ichiyama R. M., Subramanian H. H., Dutschmann M. (2010). The role of serotonin in respiratory function and dysfunction. Respir. Physiol. Neurobiol. 174, 76–88 10.1016/j.resp.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M. R., Best S., Richerson G. B. (2011). Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir. Physiol. Neurobiol. 177, 133–140 10.1016/j.resp.2011.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M. R., Richerson G. B. (2008). Interaction between defects in ventilatory and thermoregulatory control in mice lacking 5-HT neurons. Respir. Physiol. Neurobiol. 164, 350–357 10.1016/j.resp.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M. R., Richerson G. B. (2010). The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J. Appl. Physiol. 108, 1425–1432 10.1152/japplphysiol.01270.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M. R., Tattersall G. J., Harris M. B., McEvoy S. D., Richerson D. N., Deneris E. S., Johnson R. L., Chen Z. F., Richerson G. B. (2008). Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 28, 2495–2505 10.1523/JNEUROSCI.4729-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges M. R., Wehner M., Aungst J., Smith J. C., Richerson G. B. (2009). Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J. Neurosci. 29, 10341–10349 10.1523/JNEUROSCI.1963-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Ferri A. L., Milton C., Prin F., Pla P., Lin W., Gavalas A., Ang S. L., Briscoe J. (2007). Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat. Neurosci. 10, 1433–1439 10.1038/nn1985 [DOI] [PubMed] [Google Scholar]
- Johnston M. V., Fatemi A., Wilson M. A., Northington F. (2011). Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 10, 372–382 10.1016/S1474-4422(11)70016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofaso F., Dauger S., Matrot B., Vardon G., Gaultier C., Gallego J. (2007). Inhibitory effects of repeated hyperoxia on breathing in newborn mice. Eur. Respir. J. 29, 18–24 10.1183/09031936.00111705 [DOI] [PubMed] [Google Scholar]
- Madden C. J., Morrison S. F. (2010). Endogenous activation of spinal 5-hydroxytryptamine (5-HT) receptors contributes to the thermoregulatory activation of brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R776–R783 10.1152/ajpregu.00614.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S. S., Fewell J. E. (2003). Thermoregulation in rats during early postnatal maturation: importance of nitric oxide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R1366–R1372 [DOI] [PubMed] [Google Scholar]
- Maskrey M. (1990). Body temperature effects on hypoxic and hypercapnic responses in awake rats. Am. J. Physiol. 259, R492–R498 [DOI] [PubMed] [Google Scholar]
- Mortola J. P. (1999). How newborn mammals cope with hypoxia. Respir. Physiol. 116, 95–103 10.1016/S0034-5687(99)00038-9 [DOI] [PubMed] [Google Scholar]
- Mortola J. P. (2005). Influence of temperature on metabolism and breathing during mammalian ontogenesis. Respir. Physiol. Neurobiol. 149, 155–164 10.1016/j.resp.2005.01.012 [DOI] [PubMed] [Google Scholar]
- Mortola J. P., Frappell P. B. (1998). On the barometric method for measurements of ventilation, and its use in small animals. Can. J. Physiol. Pharmacol. 76, 937–944 10.1139/y99-001 [DOI] [PubMed] [Google Scholar]
- Mortola J. P., Gautier H. (1995). “Interaction between metabolism and ventilaton: effects of respiratory gases and temperature,” in Regulation of Breathing, eds Dempsey J. A., Pack A. I. (New York: Marcel Dekker; ), 1011–1064 [Google Scholar]
- Osaka T. (2010). Hypoxia-induced hypothermia mediated by noradrenaline and nitric oxide in the rostromedial preoptic area. Neuroscience 165, 976–983 10.1016/j.neuroscience.2009.10.069 [DOI] [PubMed] [Google Scholar]
- Pattyn A., Morin X., Cremer H., Goridis C., Brunet J. F. (1999). The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399, 366–370 10.1038/20700 [DOI] [PubMed] [Google Scholar]
- Pattyn A., Vallstedt A., Dias J. M., Samad O. A., Krumlauf R., Rijli F. M., Brunet J. F., Ericson J. (2003). Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev. 17, 729–737 10.1101/gad.255803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam R. W., Conrad S. C., Gdovin M. J., Erlichman J. S., Leiter J. C. (2005). Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respir. Physiol. Neurobiol. 149, 165–179 10.1016/j.resp.2005.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N., Vaubourg V., Dauger S., Matrot B., Vardon G., Chettouh Z., Gaultier C., Goridis C., Gallego J. (2006). Ventilatory response to hyperoxia in newborn mice heterozygous for the transcription factor Phox2b. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1691–R1696 10.1152/ajpregu.00875.2005 [DOI] [PubMed] [Google Scholar]
- Ramanantsoa N., Vaubourg V., Matrot B., Vardon G., Dauger S., Gallego J. (2007). Effects of temperature on ventilatory response to hypercapnia in newborn mice heterozygous for transcription factor Phox2b. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R2027–R2035 10.1152/ajpregu.00349.2007 [DOI] [PubMed] [Google Scholar]
- Ramirez J. M., Viemari J. C. (2005). Determinants of inspiratory activity. Respir. Physiol. Neurobiol. 147, 145–157 10.1016/j.resp.2005.05.003 [DOI] [PubMed] [Google Scholar]
- Renolleau S., Dauger S., Autret F., Vardon G., Gaultier C., Gallego J. (2001). Maturation of baseline breathing and of hypercapnic and hypoxic ventilatory responses in newborn mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1746–R1753 [DOI] [PubMed] [Google Scholar]
- Saiki C., Mortola J. P. (1996). Effect of CO2 on the metabolic and ventilatory responses to ambient temperature in conscious adult and newborn rats. J. Physiol. 491(Pt 1), 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawnani H., Jackson T., Murphy T., Beckerman R., Simakajornboon N. (2004). The effect of maternal smoking on respiratory and arousal patterns in preterm infants during sleep. Am. J. Respir. Crit. Care Med. 169, 733–738 10.1164/rccm.200305-692OC [DOI] [PubMed] [Google Scholar]
- Steiner A. A., Branco L. G. (2002). Hypoxia-induced anapyrexia: implications and putative mediators. Annu. Rev. Physiol. 64, 263–288 10.1146/annurev.physiol.64.081501.155856 [DOI] [PubMed] [Google Scholar]
- Waters K. A., Gozal D. (2003). Responses to hypoxia during early development. Respir. Physiol. Neurobiol. 136, 115–129 10.1016/S1569-9048(03)00076-4 [DOI] [PubMed] [Google Scholar]
- Weese-Mayer D. E., Berry-Kravis E. M., Ceccherini I., Keens T. G., Loghmanee D. A., Trang H. (2010). An official ATS clinical policy statement: congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am. J. Respir. Crit. Care Med. 181, 626–644 10.1164/rccm.200807-1069ST [DOI] [PubMed] [Google Scholar]