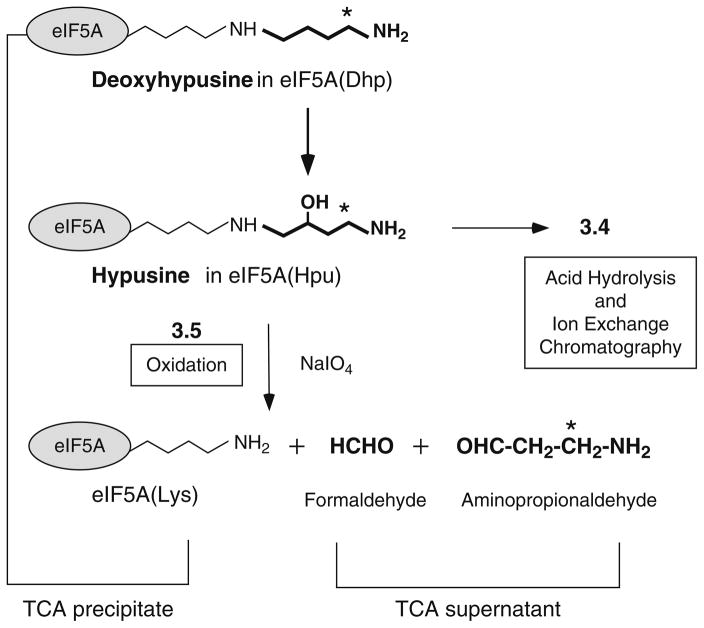
Fig. 1.
Biosynthesis of hypusine by two enzymatic steps and degradation of hypusine residue by periodate oxidation. Deoxyhypusine hydroxylase catalyzes hydroxylation of the deoxyhypusine residue at C2 of its aminobutyl side chain. Sodium (meta) periodate causes oxidative cleavages (due to vicinal amino hydroxyl groups) of the hypusine side chain to release β-propionaldehyde, which is measured as the radioactivity in the TCA supernatant, whereas the unreacted substrate protein eIF5A([3H]Dhp) is precipitated with TCA. The asterisk indicates the position of radioactivity derived from [1,8-3H]spermidine.