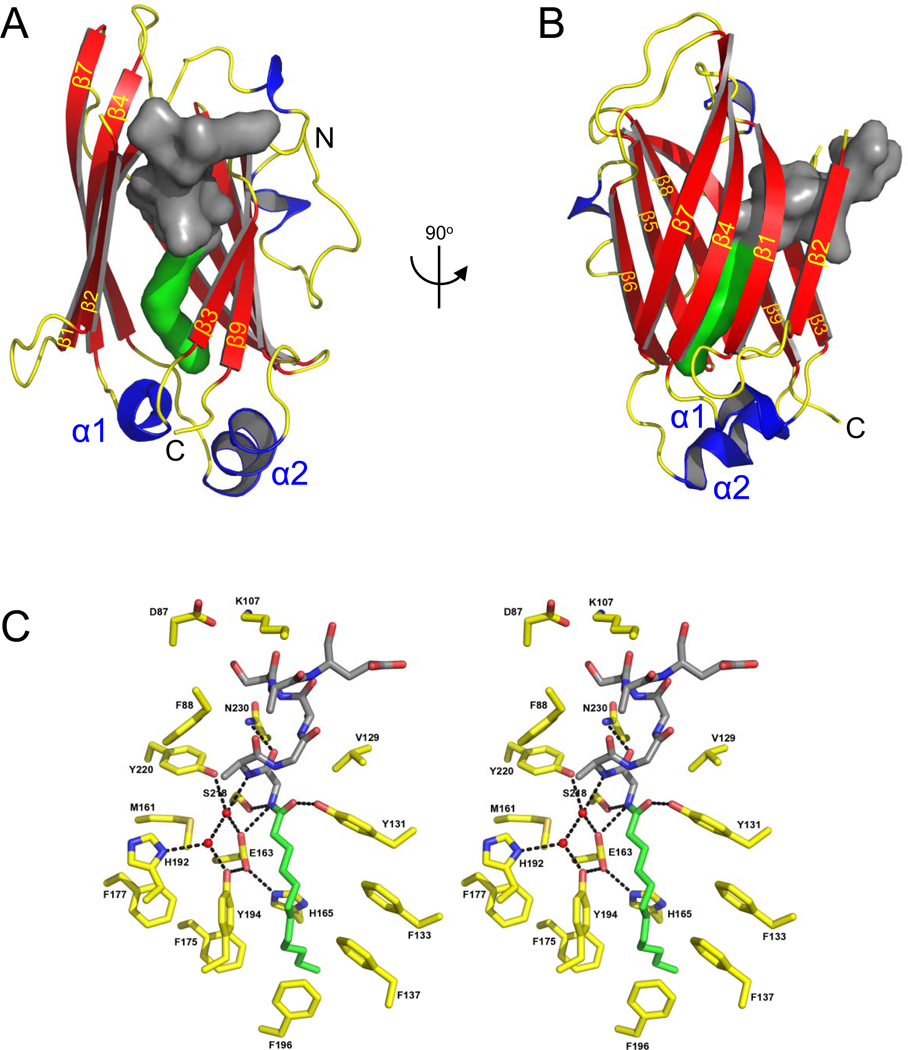
Figure 4. The lipid binding pocket of UNC119.
(A,B) Two orientations of UNC119 co-crystallized with the acylated Tα peptide in the UNC119 hydrophobic cavity. The lauroyl chain is shown in green, and the ten amino acids of the peptide are modeled in dark gray. In B, UNC119 is viewed after a 90° rotation around the vertical axis and the individual β-strands are labeled β1-9 in yellow. (C) Stereoview of UNC119 residues and key water molecules interacting with the lauroyl-GAGASAEEKH ligand. The hydrogen-bonding network (black dashed lines) limits the depth to which the Tα peptide can penetrate UNC119. Hydrogen bonds were included if the average of the bond length for all six molecules in the asymmetric unit was 3.2 Å or less and satisfied appropriate hydrogen bonding stereochemistry. UNC119 residues are shown in yellow, the lauroyl chain is green and the attached residues are colored dark gray. Figures were created with PyMOL (www.pymol.org).