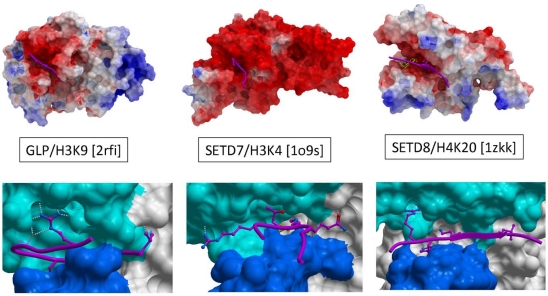
Fig. (4).
Electrostatics and chemistry of peptide recognition. Top: Electrostatic coloring (red: electronegative, blue: electropositive, gray: hydrophobic) reveals that the peptide binding groove is always electronegative, suggesting a long-range, non-specific attraction of electropositive histone tails. Bottom: Available ternary structures indicate important but distinct contribution of an arginine flanking the substrate lysine to binding enthalpy. Other residues that are also sites of post-translational modifications often occupy the binding groove.