Table 2.
Structures and activities of analogs (S)-20 and (R)-20.
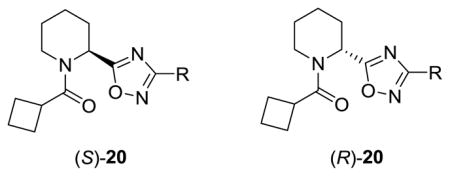 | |||||
|---|---|---|---|---|---|
| Cmpd | R | Pharmacology | IC50 (μM)a | EC50 (μMa) | Glu Max (%)a |
| (S)-20a |
|
NAM | 2.6 | NA | 11 |
| (R)-20a | NAM | 3.5 | NA | 7 | |
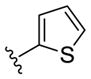
| (S)-20b |
|
NAM | 10 | NA | 33 |
| (R)-20b | Inactive | - | - | - | |
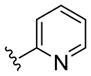
| (S)-20c |
|
PAM | NA | 0.7 | 71 |
| (R)-20c | PAM | NA | 0.6 | 37 | |
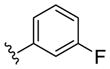
| (S)-20d |
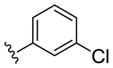
|
NAM | 0.9 | NA | 6 |
| (R)-20d | NAM | 10 | NA | 38 | |
| (S)-20e |
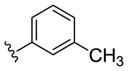
|
NAM | 0.5 | NA | 3 |
| (R)-20e | NAM | 0.3 | NA | 6 | |
| (S)-20f |
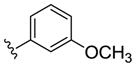
|
NAM | 0.08 | NA | 1.8 |
| (R)-20f | NAM | 0.3 | NA | 0.5 | |
Average of at least three independent determinations. NA, not applicable.
