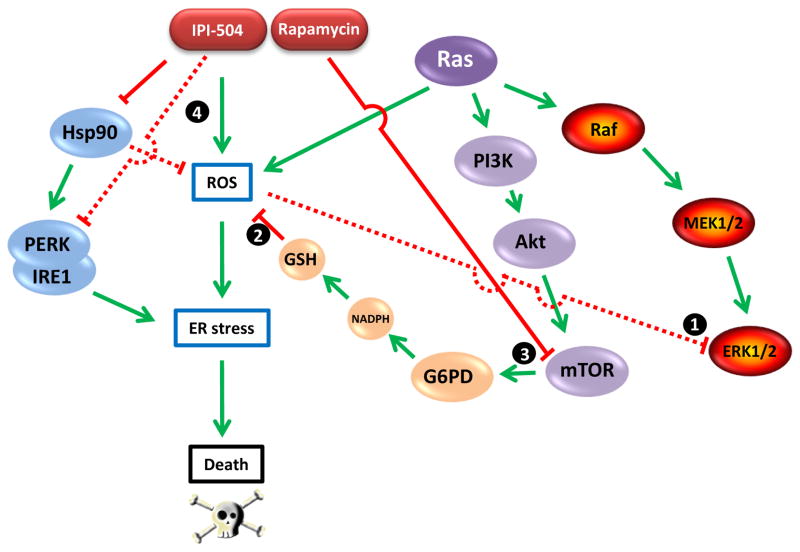
Figure 1.
The relationship between Ras-driven signaling pathways, ROS, Hsp90, and ER stress. Ras-driven proliferation requires ROS buffering of Ras-activated ERK1/2 activity (1). ROS level is regulated in part by reduced glutathione (GSH), requiring sufficient NADPH generated by G6PD-mediated glucose oxidation via the pentose phosphate pathway (2). G6PD expression is regulated by mTOR, a component of a second Ras-driven signaling pathway that is inhibited by rapamycin (3). Hsp90 buffers cellular ROS and is essential for stabilization and activity of the ER transmembrane kinases PERK and IRE1, two components of the ER stress response machinery. The Hsp90 inhibitor IPI-504 causes ROS levels to increase while also abrogating the ER stress response (4). The combination of IPI-504 and rapamycin disrupts the balance between ROS and GSH, causing catastrophic ER stress and cell death.