Abstract
Epidemiological evidence indicates that diets high in fruits and vegetables provide a measure of cancer chemoprevention due to phytochemical constituents. Natural products are a rich source of cancer chemotherapy drugs, and primarily target rapidly-cycling tumor cells. Increasing evidence indicates that many cancers contain small populations of resistant, stem-like cells that have the capacity to regenerate tumors following chemotherapy and radiation, and have been linked to the initiation of metastases. Our goal is to discover natural product-based clinical or dietary interventions that selectively target cancer stem cells, inducing differentiation. We adapted an alkaline phosphatase (AP) stain to assay plant extracts for the capacity to induce differentiation in embryonic stem (ES) cells. AP is a characteristic marker of undifferentiated ES cells, and this represents a novel approach to screening medicinal plant extracts. Following a survey of approximately 100 fractions obtained from twelve species of ethnomedically utilized plants, we found fractions from three species that induced differentiation, decreasing AP and transcript levels of pluripotency markers (Nanog, Oct-4, Rex-1). These fractions affected proliferation of murine ES, and human embryonal, prostate, and breast carcinoma cells in a dose-dependent manner. Several phytochemical constituents were isolated; the antioxidant phytochemicals ellagic acid and gallic acid were shown to affect viability of cultured breast carcinoma cells.
Keywords: Rex-1, Nanog, Oct-4, Suriana maritima, Simarouba glauca, Quassia africana
Introduction
There is increasing evidence that many cancers contain small populations of pluripotent cells capable of propagating tumors following treatment with radiation or cytotoxic chemotherapy drugs [1]. First identified in leukemia, cancer stem cells (CSCs) have since been discovered in many solid tumors, including breast, ovary, brain, melanoma, multiple myeloma, pancreas, head and neck, colon, and lung [2, 3]. It is believed that these cells have the ability to both self-renew and differentiate into tumor cells, leading to a hierarchical model that may explain the cellular heterogeneity found in many tumors. Resistance to traditional cancer chemotherapeutic drugs via the proliferation of these CSCs may also explain cancer recurrence after treatment and the subsequent initiation of metastases.
Normal stem cells are exposed to a high number of mutagenic events over time, which may in turn lead to the initiation and progression of carcinogenesis from somatic stem cells or progenitor cells [1]. The dysregulation of signaling pathways such as Notch, Shh, and Wnt contributes to self-renewal and can lead to oncogenesis [4]. Many CSCs exhibit characteristics of normal stem cells, including self-renewal, anchorage-independent growth, high cloning efficiency, and the expression of anti-apoptotic and transporter proteins [5]. Strategies that target CSCs represent a new model for cancer chemoprevention and treatment, one that aims to prevent CSCs from continuing to drive tumor growth.
Most of the currently available pharmaceutical drugs for cancer are derived from natural products [6]. Phytochemicals target a variety of cancer transcription factors such as Sp1, Sp3, c-Myc, hypoxia-inducible factor 1α (HIF-1α), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and activator protein 1 (AP-1) [7-9]. Quassinoids, polyphenolics, triterpenoids, lignans, and diterpene esters have demonstrated an ability to induce differentiation, downregulate c-Myc, and affect apoptotic pathways in stem-like cancer cells through a wide array of mechanisms [10-15].
The signaling molecule all-trans retinoic acid (RA) is currently used as a differentiation-inducing drug in combination with arsenic trioxide to successfully treat acute promyelocytic leukemia [16]. However, there are no pharmaceutical drugs currently in use for differentiation therapy against solid tumors. As the body of literature that describes and identifies CSCs grows, there is a corresponding interest in the discovery of agents that affect pluripotency and drive differentiation. Our goal is to discover natural product-based clinical or dietary interventions that selectively target CSCs. To do so, we have employed a murine embryonic stem (ES) cell line as a surrogate for CSCs, which represents a novel approach in natural products screening.
We prepared approximately 100 extracts from fifteen species (Table 1) that have either been utilized ethnobotanically or have a close taxonomic relationship to traditional botanical medicines. These extracts were assayed for their ability to induce differentiation of ES cells by alkaline phosphatase staining. Alkaline phosphatase (AP) is a characteristic marker of undifferentiated murine embryonic stem cells; the activity of the enzyme is quickly lost upon differentiation [17, 18]. In addition, cytotoxicity and anti-radical capacity of the extracts were measured. Extracts from three species (Quassia africana Baill., Simarouba glauca DC, and Suriana maritima L.) were found to reduce alkaline phosphatase activity, indicating the induction of differentiation, and were selected for cell cycle analysis by flow cytometry and mRNA analysis by semi-quantitative RT-PCR and Northern blot.
Table 1.
Plant species screened in cell viability and alkaline phosphatase assay. Species were separated into plant parts and extracted in methanol. Following the removal of the methanol, extracts were suspended in water and partitioned with hexane, ethyl acetate, and butanol to make 4 distinct fractions (including aqueous) for each plant part. Approximately 100 fractions were initially obtained and assayed for alkaline phosphatase activity and cytotoxicity.
| Family | Species | Parts used |
|---|---|---|
| Fabaceae | Lotononis bainesii | aerial parts, roots |
| Fabaceae | Sutherlandia frutescens | seeds, leaves |
| Simaroubaceae | Simarouba glauca | fruit pulp, seeds, roots, leaves, stem, bark |
| Vitaceae | Cissus quadrangularis | stems |
| Vitaceae | Cissus sp. | leaves and stems |
| Simaroubaceae | Simarouba amara | leaves, twigs |
| Irvingiaceae | Klainedoxa gabonensis | leaves |
| Simaroubaceae | Quassia africana | leaves |
| Surianaceae | Suriana maritima | leaves and stems |
| Irvingiaceae | Irvingia gabonensis | leaves |
| Asteraceae | Artemisia annua | aerial parts |
| Rutaceae | Murraya koenigii | inflorescences, leaves and stems |
| Orchidaceae | Dendrobium chrysotoxum | stem, flowers |
The species investigated further include two members of the Simaroubaceae family, Quassia africana and Simarouba glauca, as well as Suriana maritima from the allied Surianaceae family. Many species of the Simaroubaceae have historically been utilized in traditional medicinal systems around the world, and are abundant in biologically active compounds, specifically the quassinoids [19-23].
Quassia africana has been used as a treatment for malaria and as a bitter flavoring; it has been reported to contain both quassinoids and alkaloids [24-29]. Simarouba glauca (paradise tree) has been traditionally used throughout the Caribbean and South America against malaria and to treat gastrointestinal disorders. The purple fruits are edible, and the seeds, which have been included in cattle feed, contain an edible fat which can be used as a substitute for cocoa butter. Cytotoxic quassinoids, limnoids, triterpenoids, and alkaloids have been isolated from the seeds and twigs [19, 20, 22, 30].
Two flavonoid glycosides, one triterpene diol, and the plant sterol β-sitosterol have been reported from Suriana maritima [31, 32]. β-Sitosterol, a dietary supplement commonly used for prostate cancer, lowers serum low-density lipoprotein (LDL) cholesterol levels, suppresses proliferation of prostate and breast cancer cell lines, and acts as an agonist of the nuclear receptor liver X receptor (LXR), a member of the nuclear receptor superfamily that heterodimerizes with retinoid X receptor (RXR) [33].
Extracts from these three species (S. glauca, Q. africana, and S. maritima) reduced AP activity after 96 hours, had a dose-dependent activity on cell proliferation, and reduced mRNA levels of several markers of pluripotency. Based on these results, we also assayed the viability of NT-2 human embryonal carcinoma cells, androgen receptor negative (AR−) PC-3 human prostate carcinoma cells, and estrogen receptor α positive (ER+) MCF-7, and estrogen receptor α negative (ER−) SK-BR-3 human breast adenocarcinoma cells treated with the extracts. We found that they displayed a differential, dose-dependent effect on the cancer cell growth for each cell line. The major constituents were subsequently isolated or identified and assayed when possible.
Materials and Methods
Plants
Dried leaves and stems of Suriana maritima and Quassia africana were a gift of Dr. Wayt Thomas, The New York Botanical Garden (NYBG). Bare-root Simarouba glauca saplings were purchased live from Garden of Delights (Davie, FL). The remaining plants were received from Dr. Brad Morris of the Plant Genetic Resources Conservation Unit, USDA; Matt Johnson of the Desert Legume Program, UDSA; Barbara Whiehe of Ohio Weslyan University; Clinton Morse of University of Connecticut; Colleen Armstrong of the University of Vermont; David Cain of Lehman College, City University of New York (CUNY), or collected by K.A.R. near the John D. MacArthur State Park in West Palm Beach, FL.
Extraction
Plants were separated into roots, stems, fruits, seeds, leaves, and twigs and each part was lyophilized prior to extraction with 80% aqueous methanol. Extracts were concentrated under reduced pressure in a rotary evaporator, diluted with water, and partitioned sequentially with hexane, ethyl acetate, and butanol to give 4 fractions (including the aqueous layer). These fractions were dried under nitrogen, lyophilized, and stored at −20 °C. Extracts were dissolved in dimethyl sulfoxide (≤ 50 μg/ml) and diluted in the culture medium (dimethyl sulfoxide concentration ≤ 0.1%) for cell culture experiments.
Cell culture
Murine AB1 ES cells were cultured in monolayer at 5% CO2 in Dulbecco's Modified Essential (DME) Medium with 10% fetal calf serum and 1 × 103 units/ml leukemia inhibitory factor (LIF) (Millipore-Chemicon) for all experiments. PC-3 human prostate carcinoma cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 supplemented with 10% fetal calf serum at 10% CO2. NT-2 embryonal carcinoma cells, MCF-7 and SK-BR-3 human breast adenocarcinoma cells were cultured in DME with 10% fetal calf serum at 5% CO2. For all cell culture experiments, cells were allowed to attach prior to addition of treatments. Thereafter, treatments and media were changed at 48 hrs for 96-hour protocols or 72 hrs for 5- and 6-day protocols.
Alkaline Phosphatase Differentiation Assay
Cells were plated at a concentration of 1000 cells/well in gelatinized 96-well plates and allowed to adhere prior to treatment. Treatments and media were changed at 48 hrs. Alkaline phosphatase (AP) activity was visualized by Fast Red (Sigma) staining, and monitored by light microscopy after 96 hrs. AP activity is quickly lost upon differentiation [17]. The signaling molecule all-trans retinoic acid is a potent inducer of ES cell differentiation and was used as a positive control [34]. Vehicle (≤0.1% dimethyl sulfoxide) was used as the negative control.
WST-1 Cytotoxicity/Cell Proliferation Assay
Cells were plated at a concentration of 1000 to 1500 cells/well in gelatinized 96-well plates and allowed to adhere prior to treatment. Treatments and media were changed at 48 hrs for 96-hr protocols. Cytotoxicity/cell proliferation was measured using the water-soluble tetrazolium salt WST-1 (Roche). The WST-1 salt is cleaved by succinate-tetrazolium reductase in the mitochondria to form a water-soluble formazan dye; this bioreduction occurs primarily at the cell surface and is largely dependent on the glycolytic production of NAD(P)H in viable cells [35, 36]. Cell proliferation was measured at 48 and 96 hrs. WST-1 is added at a concentration of 1:10, and incubated at 37 °C for approximately 1 hour; the absorbance measured at 450 nm is directly proportional to the number of metabolically active cells when compared to the vehicle control.
Trypan Blue Exclusion Assay
Murine ES cells were plated at a concentration of 6 × 105 (18 h), 3 × 105 (48 h), and 1 × 105 (96 h) cells/well in gelatinized 6-well culture plates. Treatments and media were changed at 48 hrs for 96-hr treatments. Cells were trypsinized, centrifuged, and resuspended in 2 ml media. A cell suspension was mixed with equal parts trypan blue dye (Gibco), and placed in a haematocytometer to count stained and unstained cells. Metabolically active cells will exclude the dye.
Flow Cytometry
Murine ES cells were plated at a concentration of 3 × 105 cells/plate in gelatinized 100 mm culture plates. Treatments and media were changed at 48 hrs. At 96 h, cells were trypinized, centrifuged, washed with PBS, and fixed with 70% ethanol. After fixation, cells were washed with PBS, and stained with a propidium iodide (PI) solution containing 50 μg/ml PI (Sigma) and 100 μg/ml RNase A (Sigma) in PBS. The DNA content of 100,000 cells was measured with a Becton Dickinson FACScan flow cytometer and the data analyzed with FlowJo software cell cycle analysis tool.
M Antiradical Assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay is a rapid colorimetric screen for antioxidant activity used widely in natural products research [37-39]. Serial dilutions in dimethyl sulfoxide or methanol were combined with an ethanolic DPPH (400 μM) solution in 96-well microtiter plates and incubated for 30 min at 37 °C. The change in absorbance at 517 nm was measured to calculate a DPPH IC50. Dimethyl sulfoxide or methanol was used as a negative control and gallic acid (IC50 = 30.0 ± 2.9 μM) was used as a positive control.
RT-PCR analysis
For assays of mRNA levels, murine ES cells were plated at a concentration of 3 × 106 (18 h), 1.5 × 106 (48 h), and 2.5 × 105 (96 h) cells/plate in gelatinized 100 mm culture plates. Treatment concentrations reflect the approximate the IC50 of each extract (see Figure 2 caption). Treatments and media were changed at 48 hrs. Cells were harvested with Trizol (Invitrogen) and quantified by measuring optical density at 260 nm. mRNA (5 μg) was reverse transcribed to cDNA and diluted ten-fold. PCR reactions utilized 2 μl of cDNA. PCR primers were designed to span introns and checked against the UCSC in-silico PCR database to minimize the amplification of genomic DNA or pseudogenes. The housekeeping gene 36B4 was used to assure equal loading among samples [40].
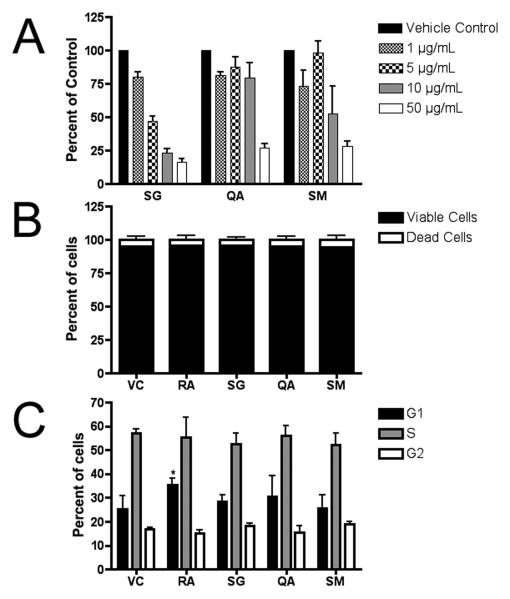
Figure 2. (A) Cytotoxicity/cell viability of murine embryonic stem cells following 96 hr treatment.
Each fraction demonstrated a dose-dependent effect on cell proliferation in the WST-1 assay. Experiments were performed in duplicate wells, and 3 independent biological replicates were assayed (mean ± SE) and plotted relative to the absorbance (450 nm) of the untreated control (0.1% DMSO). From these experiments, approximate IC50 values were determined (BuOH fraction of wood extract from Simarouba glauca saplings (SG) IC50 = 2.5 μg/ml; aqueous fraction of leaf extracts from Quassia africana (QA) IC50 = 25 μg/ml; etOAc fraction of leaf and stem extracts from S. maritima (SM) IC50 = 12.5 μg/ml); Vehicle control (VC) = 0.1% DMSO. (B) Trypan blue exclusion assay. There was no significant difference in the ratio of living to dead cells after 96 h treatments as compared to 1 μM retinoic acid-treated (RA) or vehicle controls (VC). Cells were tallied in 3 independent biological experiments (mean ± SE). (C) Cell cycle analysis. Propidium iodide-stained ES cells were analyzed by flow cytometry. RA significantly increased the number of cells in G1 (T-test; p<0.05), however, no significant differences were measured between treatment and control groups. (n=3; mean ± SD).
Commercial Taq polymerase (Invitrogen or Denville) was used for PCR reactions, which were run on an MJ Research PTC-100 or BioRad iCycler as follows: 95 °C initial denaturation (5 min); denature at 94 °C for 30 s; anneal at 58 – 65 °C (based on primer pair) for 30 s; extension at 72 °C for 45 s; the optimum number of cycles in the linear range was determined for each primer pair to prevent saturation. PCR products were separated using ethidium bromide-stained agarose gel electrophoresis. Densitometry of bands was measured with ImageJ software (NIH).
Murine primer sequences are as follows:
| Gene | Forward | Reverse |
|---|---|---|
| 36B4 | AGAACAACCCAGCTCTGGAGAAA | ACACCCTCCAGAAAGCGAGAGT |
| BMP4 | CCCAGAAGCAGCTGCTGGCGA | GCAGGAGCTCATGGCTCTGCCCT |
| Cdh1 | GATGCCCGACCGGAAGTGACT | TCTACACACTCAGGGAAGGAGCTGA |
| Cdh2 | CATCAAGCCCGTGGGAATCAGA | AGCCAAGTCCGTCCTGCCGT |
| GATA6 | ATGGCGTAGAAATGCTGAGG | TGAGGTGGTCGCTTGTGTA |
| HNF1b | GAAAGCAACGGGAGATCCTCCGAC | TAGGCATCCATGGCCAGCTTCTGC |
| LamB1 | GCAGACACAACACCAAAGGC | TGTACCCATCACAGATCCCG |
| ID1 | CTGGAGCTGAACTCGGAGTCTGAA | GCCGCCTCAGCGACACAAGAT |
| Meis1 | CATGATAGACCAGTCCAACC | GGCTACATACTCCCCTGGCATACT |
| MEST | CAATCCTGCGGCGGGCGGCATGGGA | GGTAGAAGATGCGTAGGCCTTTGTAGGTGA |
| Nanog | AAAGGATGAAGTGCAAGCGGTGG | CTGGCTTTGCCCTGACTTTAA |
| Oct-4 | GGCGTTCTCTTTGGAAGGGTGTTC | CTCGAACCACATCCTTCTCT |
| Pax6 | AGACTTTAACCAAGGGCGGT | TAGCCAGGTTGCGAAGAACT |
| PDGFRα | CGTGGAAATCAGAAGTGAGGAGA | ACGCTGAAGGTTCCGTTGAAG |
| Rex-1 | GAAAGCAGGATCGCCTCACTGTGC | CGATAAGACACCACAGTACACAC |
Northern Blot Analysis
mRNA was isolated with Trizol (Invitrogen), and quantified by measuring optical density at 260 nm. Northern Blot analysis was performed as previously described using 10 μg mRNA [41]. Signal intensity was quantified using a phosphorimager.
Compound Isolation
Extracts of S. maritima and S. glauca were separated by column chromatography using Sephadex LH-20 (Pharmacia Fine Chemicals; 25-100 μm) and isocratic solvent systems consisting of methanol or (1:1) methanol/water; reversed-phase C18 silica gel (J. T. Baker; 40 μm) and a gradient solvent system from 95% water to pure methanol in 5% steps; and silica gel (J. T. Baker; 60 Å) with a gradient solvent system of hexane/ethyl acetate/methanol (100:0:0 to 0:100:0 to 0:0:100 in 25% steps). Fractions were analyzed by HPLC-PDA, LC-MS (conditions below), and thin-layer chromatography (Merck; silica gel 60 F254; 1 mm thickness) with a (8:2:1) chloroform/methanol/formic acid solvent system.
Compound Identification
Structures of pure compounds were established by NMR and LC-MS comparison with authentic standards or literature values [19, 31]. NMR spectra were collected on a Bruker Avance AV500 spectrometer operating at 500 MHz for 1H and 125 MHz for 13C using standard Bruker TopSpin software. Mass spectra were measured with a Waters Acuity UPLC/MS in ESI positive and negative modes using a Waters Acuity BEH C18 column (2.1 × 30 mm; 1.7 μm); the solvent system consisted of a gradient elution (95 to 60% A; 0.6 ml/min; 8 min) of 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). HPLC-PDA was performed on a Waters 2695 using a Phenomenex Synergi Fusion-RP column (250 × 4.6 mm, 4 μm) and monitored with a Waters 996 PDA scanning from 240 to 600 nm and a gradient solvent system (95 to 60% A; 1 ml/min; 30 min) of 5% formic acid (A) and acetonitrile (B). Rutin, ellagic acid, and gallic acid were purchased from Sigma (MO, USA).
Statistical Analysis
Experiments were performed in triplicate, and calculations of the means, standard deviations, standard error, and measurements of statistical significance (ANOVA or T-test; P < 0.05) were performed using the statistical software Prism (v. 4.0).
Results
Alkaline phosphatase activity of embryonic stem cells is reduced by plant extracts
Murine ES cells are typically maintained in an undifferentiated state by the addition of LIF to the culture medium; LIF was used in all mES cell culture experiments. Expression of alkaline phosphatase (AP) is high in pluripotent stem cells, and decreases rapidly as cells initiate differentiation [17, 18]. We therefore used AP activity as a screen to determine if any of the nearly 100 fractions obtained induced differentiation in murine ES cells. After a 96-hour treatment, cells cultured in media with extracts from three species lost AP activity. All-trans retinoic acid (RA) induces ES cells to differentiate, and was used as the positive control [42].
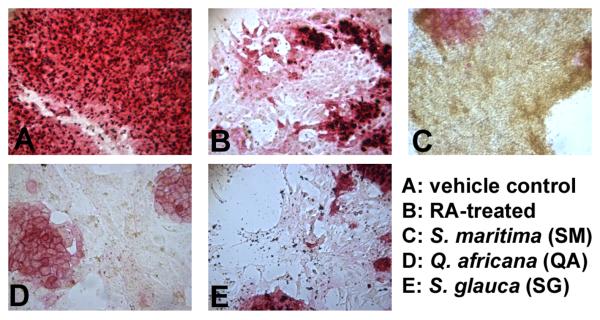
The butanol fraction of wood extract from S. glauca saplings (SG), the ethyl acetate fraction of leaf and stem extracts from S. maritima (SM), and the aqueous fraction of leaf extracts from Q. africana (QA) induced differentiation as detected by a reduction or absence in AP staining. Cells treated with these extracts for 96 hrs were morphologically distinct from untreated, undifferentiated cells; RA-treated positive controls for cell differentiation [34]; and each other (Figure 1). Cells treated with SM (50 μg/ml) continued to proliferate, but lost AP activity, developing a shrunken, round shape and orange color after 96 hours of treatment. SG (5 μg/ml) caused cells to elongate; higher concentrations proved cytotoxic. Cells treated with QA (50 μg/ml) lost AP activity and grew larger and showed a more flattened morphology. Fractions from the remaining plant extracts did not induce differentiation by AP stain, and many were either toxic at concentrations above 100 ng/ml or non-toxic up to 50 μg/ml.
Figure 1. Alkaline phosphatase staining of murine embryonic stem cells.
Cells were treated with semi-purified fractions of plant extracts. Fractions were dried completely, resuspended in DMSO, and diluted in culture medium to final concentrations (1, 5, 10 and 50 μg/ml) and a DMSO concentration ≤ 0.1%. After 96 h treatment, cells were stained and compared to (A) vehicle control (0.01 % DMSO) and (B) 1 μM RA-treatment. Alkaline phosphatase activity decreases rapidly with the onset of differentiation, which can be seen in B-E. Pictured are cells treated with (C) the ethyl acetate fraction of leaf and stem extracts of Suriana maritima (50 μg/ml); (D) the aqueous fraction of leaf extracts from Q. africana (50 μg/ml); and (E) the butanol fraction of wood extract from S. glauca saplings (5 μg/ml). Experiments were performed in duplicate wells with at least 3 independent biological replicates.
Extracts showed a dose-dependent effect on proliferation of murine embryonic stem cells
Cytotoxicity/cell viability was measured using the WST-1 assay (Figure 2A). Approximate IC50 values of SG, SM and QA were determined (2.5 μg/ml, 12.5 μg/ml, and 25 μg/ml, respectively) and used to treat cells for further analyses. Utilizing the trypan blue exclusion assay, we determined that the semi-purified fractions caused growth arrest rather than a cytotoxic response at the IC50 values. We found a dose-dependent reduction in cell number after 96 hrs without a significantly higher percentage of dead or dying cells when compared to the vehicle control (Figure 2B). Flow cytometery of propidium iodide-stained cells, however, did not reveal any significant cell cycle differences between the controls and the fraction-treated cells, whereas RA treatment significantly increased the percentage of cells in G1 (Figure 2C).
Analysis of pluripotency marker transcript levels altered by extracts
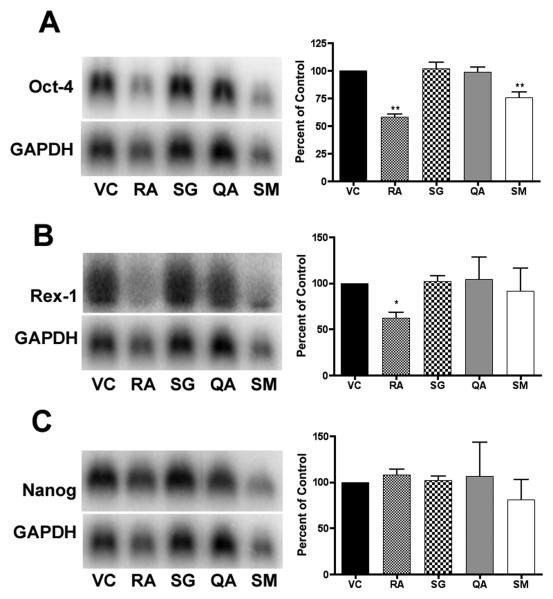
In order to investigate the changes in differentiation state further, we performed Northern blot (Figure 3) and semi-quantitative RT-PCR (Figure 4) analysis of transcript levels of stem cell markers Rex-1 (Zfp-42), Nanog, and Oct-4 [42-44]. Northern blot analysis indicated a significant decrease (p < 0.01) in Oct-4 transcript levels in both the RA- and SM-treated ES cells at 96 h. A significant decrease (p < 0.01) in Oct-4 transcript levels can also be seen in SM-treated ES cells at 18 and 48 hrs, and in the RA-treated ES cells at 48 hrs. Nanog mRNA levels decreased significantly in SM-treated ES cells at 18 (p < 0.01) and 48 hrs (p < 0.05) and approached significance at 96 hrs. Rex-1 mRNA levels were also significantly decreased in cells treated with SM at 18 (p < 0.01) and 48 hrs (p < 0.05), and in cells treated with RA at 48 (p < 0.01) and 96 hrs (p < 0.05).
Figure 3. Northern blot analysis.
RNA was harvested from murine ES cells following 18, 48 and 96 hr treatments. Experiments were performed in triplicate, with three independent RNA samples, and showed similar results. Representative blots of 96 hr treatments are shown. Bands were measured by densitometry using a phosphoimager, averaged, normalized to GAPDH, and plotted (± SD) as a percent of the vehicle control. Significance is indicated by one (p < 0.05) or two asterisks (p < 0.01).
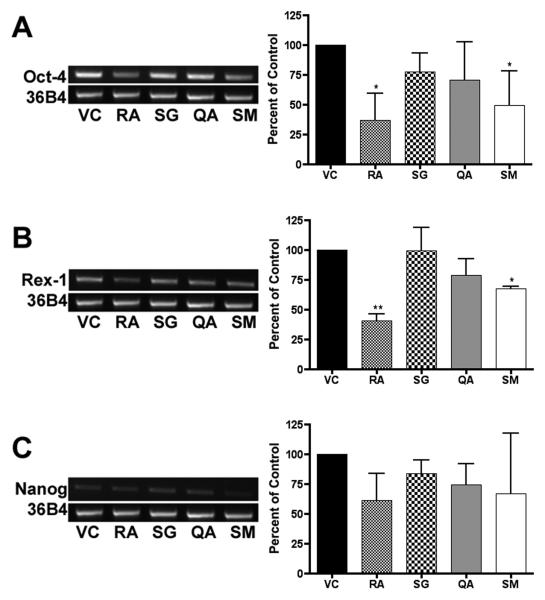
Figure 4. RT-PCR analysis.
RNA was harvested from murine ES cells at 18, 48, and 96 hrs, reverse transcribed to cDNA, and used for RT-PCR reactions. Experiments were performed in triplicate with three independent RNA samples, and showed similar results. Representative gels of 96 hr treatments are shown for transcript levels of pluripotent markers Oct-4 (26 cycles), Rex-1 (27 cycles), and Nanog (26 cycles). Cycle number was optimized using serial dilutions to prevent saturation. Gel bands were measured by densitometry, averaged, normalized to 36B4, and plotted (± SD) as a percent of the vehicle control. Significance is indicated by one (p < 0.05) or two asterisks (p < 0.01).
RT-PCR analysis of the ES cells treated for 96 hrs yielded results similar to the Northern Blot analysis. Oct-4 transcript levels were significantly decreased in cells treated with SM and RA (p < 0.05). Rex-1 mRNA levels were significantly decreased by SM (p < 0.05) and RA (p < 0.01).
Influence of extracts on specific lineage pathways
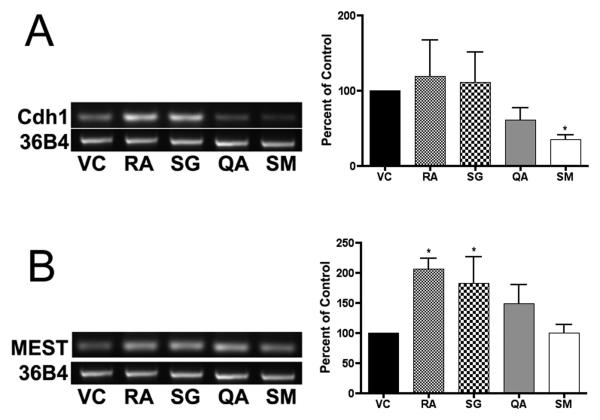
The mRNA levels of several genes involved in lineage determinations were measured by RT-PCR to determine if the extracts induced specific epithelial differentiation pathways (Figure 5). E-cadherin (cdh1) mRNA levels were significantly lower (p < 0.05) in cells treated for 96 hrs with SM; RA increased n-cadherin (cdh2) in ES cells at 48 h (p < 0.05); SG and RA increased mesoderm-specific transcript homolog (MEST) mRNA levels at 48 h (p < 0.05); RA increased meis homeobox 1 mRNA levels at 48 h (p < 0.05) and 96 h (p < 0.01); RA increased hepatocyte nuclear factor 1 homeobox B (HNF1b) mRNA levels at 18 (p < 0.05) and 48 h (p < 0.01). Fractions from Q. africana (QA) did not alter the levels of these epithelial differentiation-associated mRNAs. Transcript levels of the genes GATA6, Paired box gene 6 (PAX6), platelet-derived growth factor receptor alpha (PDGFRα), and bone morphogenetic protein (BMP4) did not significantly change after treatment with the plant extracts for up to 96 hrs (data not shown).
Figure 5. Lineage RT-PCR analysis.
RNA was harvested from murine ES cells at 18, 48, and 96 hrs, reverse transcribed to cDNA, and used for RT-PCR reactions. Experiments were performed in triplicate with three independent RNA samples, and showed similar results. Representative gels of (A) cdh1 (33 cycles) following 96 hr treatment, and (B) MEST (33 cycles) following 48 hr treatment are shown. Cycle number was optimized using serial dilutions to prevent saturation. Gel bands were measured by densitometry, averaged and plotted (± SD) relative to 36B4. Significance is indicated by an asterisk (p < 0.05).
Isolation and identification of major constituents
The fractions under investigation are complex mixtures of natural products; it was not clear which compounds were responsible for the decrease in AP activity and the changes in gene expression. This is the first report of extracts from these plants altering the levels of specific transcripts in mammalian cells. We therefore used column chromatography to separate and purify the major constituents.
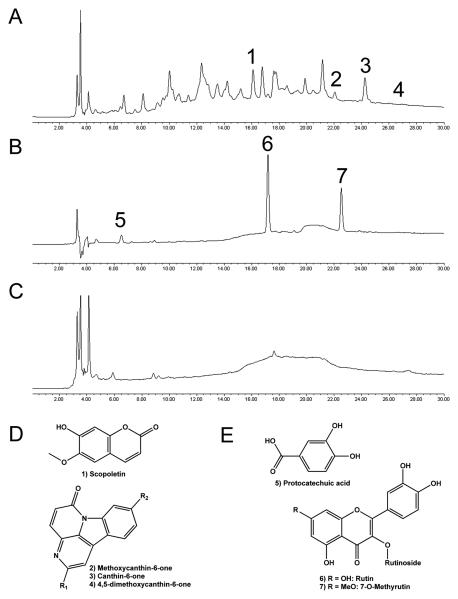
Small amounts of scopoletin, canthin-6-one, and a canthin-6-one dimethoxy derivative were isolated from the wood extract of S. glauca saplings (Figure 6A and 6D) and identified by comparison with mass spectra and literature values [45]. In addition, two canthin-6-one methoxy derivatives were identified by LC-MS, but not isolated.
Figure 6. Compounds identified in selected medicinal plants.
HPLC-PDA chromatogram at 254 nm of (A) BuOH fraction of wood extract from Simarouba glauca saplings (SG); (B) etOAc fraction of leaf and stem extracts from S. maritima (SM); (C) aqueous fraction of leaf extracts from Quassia africana (QA) IC50 = 25 μg/ml. Chemical structures of compounds identified in (D) SG and (E) SM. HPLC conditions are described in the Materials and Methods section.
The phenolic compounds rutin, 7-O-methylrutin, and protocatechuic acid were isolated and identified as the three main constituents of the ethyl acetate fraction of leaf and stem extracts of Suriana maritima (SM) (Figure 6B and 6E). Compound identification was based on mass spectra and NMR comparisons with published values or authentic standards [31, 37]. Rutin and 7-O-methylrutin have been previously found in this species [31], but this is the first report of protocatechuic acid occurrence. Pure isolates of rutin, 7-O-methylrutin, and protocatechuic acid had minimal effects on AP staining, and in the WST-1 cytotoxicity assay they displayed IC50 values of approximately 50 μM.
The aqueous fraction of leaf extracts from Q. africana (QA) was not subjected to further separations because of a lack of sufficient plant material. While canthin-6-one alkaloids have been previously reported from Q. africana extracts, they are mid- to nonpolar, and are unlikely to be are present in aqueous fractions , which contain a few very polar constituents (Figure 6C).
Effects of extracts and phytochemicals on cultured human prostate and breast cancer cells
We treated several human cancer cell lines with the SG, QA, and SM fractions, and found dose-dependent effects on cell proliferation with PC-3 human prostate carcinoma cells, NT-2 human embryonal carcinoma cells (Figure 7), and SK-BR-3 and MCF-7 human breast adenocarcinoma cells treated for 5 or 6 days (Figure 8). We then isolated several pure compounds from the SG and SM fractions. Rutin, 1-O-methylrutin, and scopoletin from SG and SM were used to treat breast cancer cells to measure cytotoxicity. Scopoletin showed moderately high cytotoxicity against MCF-7 and SK-BR-3 cells, with an IC50 of 9.1 and 28.8 μM, respectively (data not shown). Rutin and 7-O-methylrutin were inactive against both cell lines in cytotoxicity assays at concentrations up to 50 μM.
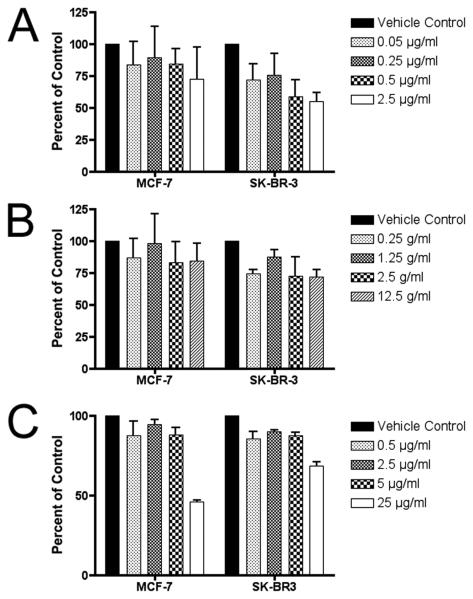
Figure 7. Inhibition of proliferation of NT-2 and PC-3 human carcinoma cell lines treated with plant extracts.
(A) NT-2 embryonal carcinoma cell proliferation is affected in a dose-dependent manner when treated with plant extracts at concentrations similar to murine ES cells. (B) PC-3 human prostate carcinoma cells were less growth-inhibited by the treatments. Cells were treated for 5 days, and viability measured using the WST-1 assay, in duplicate wells with 3 independent biological replicates, and plotted (mean ± SE) relative to the absorbance (450 nm) of the untreated control (0.1% DMSO).
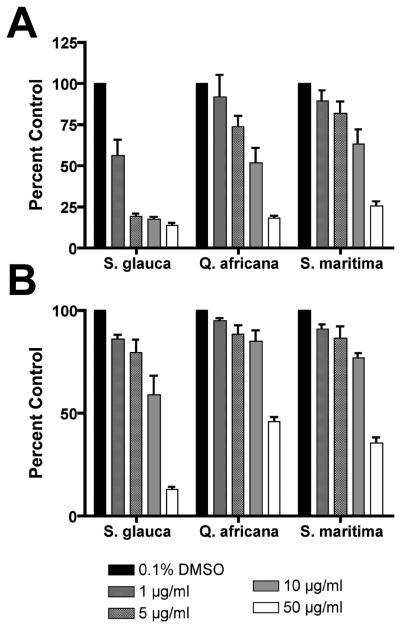
Figure 8. Inhibition of proliferation of MCF-7 and SK-BR-3 human carcinoma cell lines treated with plant extracts.
Breast adenocarcinoma cell lines MCF-7 and SK-BR-3 also show some dose-dependent activity when treated with (A) the BuOH fraction of wood extract from Simarouba glauca saplings; (B) the etOAc fraction of leaf and stem extracts from S. maritima; and (C) the aqueous fraction of leaf extracts from Quassia africana. Cells were treated for 6 days, and viability measured using the WST-1 assay, in duplicate wells with 3 independent biological replicates, and plotted (mean ± SE) relative to the absorbance (450 nm) of the untreated control (0.1% DMSO).
In additional experiments, breast cancer cells were treated with the antioxidant phytochemicals gallic acid and ellagic acid. While these compounds were not identified in the test species, they are fairly ubiquitous in higher plants and plant-based diets. Ellagic acid and ellagitannin-rich blueberry extracts were recently shown to inhibit Wnt signaling [46], and inhibit breast cancer cells though modulation of the Pi3K/AKT/NFκB pathway [47]. Ellagic acid was cytotoxic against SK-BR-3 cells (IC50 = 14.7 μM) but inactive against MCF-7 cells. Gallic acid was less active with an IC50 of 49.1 μM (MCF-7) and 63.9 μM (SK-BR-3) (data not shown).
Discussion
The Fast Red alkaline phosphatase stain is commonly used to determine the pluripotency of cells and colonies in culture. We have devised a 96-well assay that can be used to simultaneously assay multiple concentrations of several plant extracts or purified natural products rapidly. When done in tandem with a cell viability assay using the tetrazolium salt WST-1, the combination in duplicate plates can be used for bioactivity-guided fractionation to identify natural products with the potential to overcome LIF and induce differentiation in pluripotent murine ES cells while also monitoring the corresponding cytotoxicity. In doing these assays together, we were able to distinguish a cytotoxic response from an induction of differentiation.
Utilizing these assays, we found three fractions out of 100 (3%) that reduced AP activity after 96 hr treatments at concentrations that were not highly cytotoxic. Most notably, ethyl acetate fractions of S. maritima leaf and stem extracts reduced the mRNA levels of putative stem cell markers Oct-4, Nanog and Rex-1 at 18, 48, and 96 hrs. This extract affected the proliferation of murine ES cells, and human embryonal, prostate and breast cancer cells in a dose-dependant manner. Upon examination of transcript levels of several lineage markers, we found that in comparison with vehicle controls, this extract also reduced mRNA levels of the epithelial cell adhesion marker e-cadherin. A comprehensive understanding of the phytochemical constituents of these extracts is essential to unraveling the mechanisms of action. In addition, because these plants contain polyphenolic compounds common in plant-based diets, a chemical profile may have implications for dietary cancer chemoprevention.
As expected, the ethyl acetate fractions of S. maritima contain mid-polar compounds, including rutin, 7-O-methylrutin, and protocatechuic acid as the major constituents. Rutin and protocatechuic acid are well-known antioxidant polyphenols with cancer chemopreventive activity [48, 49]. They likely contribute greatly to the overall potent antioxidant activity of the fraction, which was found to have an IC50 = 16 μg/ml in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. None of these isolated compounds induced a significant AP decrease individually at concentrations up to 81 μM, which corresponds to 50 μg/ml; this is much less potent than the parent fraction, which induced differentiation at concentrations as low as 12.5 μg/ml. The pure compounds also had less effect on ES and breast cancer cell growth, with IC50 values approximately 4 times higher than the parent fraction. Because the 3 main compounds isolated from S. maritima etOAc fractions are well-known and fairly ubiquitous in plant-based foods, we feel it is unlikely that they are responsible for the decrease in AP activity. Based on these results, we conclude that the identities of the differentiation-inducing compounds in S. maritima are as yet unidentified minor compounds, and this plant requires more investigation. The phytosterol β-sitosterol has been previously isolated from this species; however, it was not detected in the extract we used for these experiments. Slightly more non-polar, it likely partitions into the hexane fraction.
While fractions of Q. africana and S. glauca decreased the AP activity, they did not significantly affect transcript levels of pluripotency markers at 18, 48 or 96 hrs. The fraction of S. glauca tested did significantly increase MEST mRNA transcript levels at 48 h in comparison to vehicle controls. This may indicate an early induction of a mesodermal lineage. However, transcript levels of brachyury and PDGFRα, also mesodermal markers, were not significantly altered by 96 hrs. Both extracts altered the morphology of ES cells and demonstrated a dose-dependent effect on cell proliferation. The butanol fraction of SG was active in the DPPH anti-radical assay (IC50 = 30 μg/ml) and the aqueous fraction of QA was inactive (IC50 > 200 μg/ml).
The butanol fraction of S. glauca was dominated by the alkaloids canthin-6-one, its derivatives, and a host of small polar compounds. The canthin-6-one alkaloids have antifungal activity [50, 51] and are cytotoxic against P-388 leukemia [52], MCF-7 breast, and A-549 lung cancer lines [53]. We also identified the coumarin scopoletin, an anti-radical scavenging compound with anti-inflammatory activity [54-56]. We found it to be the most cytotoxic compound against MCF-7 and SK-BR-3 cells. Its cytotoxic activity was similar to the parent fraction in both MCF-7 (9 μM IC50 corresponds to approximately 2 μg/ml, compared to 5.5 μg/ml for the parent SG fraction) and SK-BR-3 cells (28 μM IC50 corresponds to approximately 5.5 μg/ml, compared to 3.3 μg/ml for the parent SG fraction). However, in the parent fraction, scopoletin was one of many compounds. The canthin-6-one alkaloids were not isolated in sufficient quantity to perform cell viability assays at this time, and many minor compounds remain unidentified.
We have demonstrated that a 96-well plate alkaline phosphatase stain can be utilized as a rapid and effective assay for the evaluation of medicinal plant extracts with the ability to induce differentiation in murine ES cells. Together with a cell viability assay like the WST-1 assay, the cytotoxicity of various concentrations can be monitored to insure that the lack of AP staining is not due to cell death. Furthermore, this assay can be used in a bioactivity-guided fractionation program to isolate compounds from complex plant extracts with differentiation-inducing activity. The murine ES cells utilized in this assay grow rapidly, are well-characterized, and do not need a mouse embryonic fibroblast feeder layer to grow. Promising compounds identified by this assay can be moved forward into more complex and expensive mechanistic assays, or in future investigations against pluripotent cancer cells derived from primary tissue or from cancer stem cell lines as they become available.
Supplementary Material
Acknowledgements
Support was provided in part by NIH-NCCAM grant # T32AT001161, Department of Defense CDMRP Breast Cancer Research Program postdoctoral fellowship # W81XWH-09-1-0606, and funds from WCMC. Invaluable technical assistance and scientific discussions were provided by Carlos Rodriquez, Naira C. Rezende and Dr. Xiao-Han Tang in the Gudas lab. Drs. Nigel P. Mongan, Kristian B. Laursen, and Kymora Scotland are thanked for reagents and scientific discussions. Plant material used in this study was supplied by Dr. Wayt Thomas (NYBG), Matt Johnson and Dr. Brad Morris (USDA), Barbara Whiehe (Ohio Weslyan University), Clinton Morse (University of Connecticut), Colleen Armstrong (University of Vermont), and David Cain (Lehman College, CUNY; presently at Cornell University, NYSAES).
Abbreviations
- AP
alkaline phosphatase
- AP-1
activator protein 1
- AR
androgen receptor
- CSC
cancer stem cell
- DMSO
dimethyl sulfoxide
- ER
estrogen receptor
- ES
embryonic stem
- EtOAc
ethyl acetate
- etOH
ethanol
- HIF-1α
Hypoxia-inducible factor-1α
- LIF
leukemia inhibitory factor
- LXR
liver X receptor
- MeOH
methanol
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PI
propidium iodide
- QA
aqueous fraction of leaf extracts from Q. africana
- RA
all-trans retinoic acid
- RXR
retinoid X receptor
- SG
BuOH fraction of wood extract from S. glauca saplings
- SM
etOAc fraction of leaf and stem extracts from S. maritima
- VC
vehicle control
- WCMC
Weill Cornell Medical College
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J. Clin. Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginestar C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha M, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visvander JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Can. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 4.Schugar RC, Robbins PD, Deasy BM. Small molecules in stem cell self-renewal and differentiation. Gene Ther. 2007;15:126–135. doi: 10.1038/sj.gt.3303062. [DOI] [PubMed] [Google Scholar]
- 5.Tokar EJ, Ancrile BB, Cunha GR, Webber MM. Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation. 2005;73:463–473. doi: 10.1111/j.1432-0436.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- 6.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. Journal of Natural Products. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 7.Kaur M, Agarwal R. Transcription Factors: Molecular Targets for Prostate Cancer Intervention by Phytochemicals. Curr. Cancer Drug Targets. 2007;7:355–367. doi: 10.2174/156800907780809732. [DOI] [PubMed] [Google Scholar]
- 8.Touillaud MS, Pillow PC, Jakovljevic J, Bondy ML, Singletary SE, Li D, Chang S. Effect of dietary intake of phytoestrogens on estrogen receptor status in premenopausal women with breast cancer. Nutr. Can. 2005;51:162–169. doi: 10.1207/s15327914nc5102_6. [DOI] [PubMed] [Google Scholar]
- 9.Limer J, Speirs V. Phyto-oestrogens and breast cancer chemoprevention. Breast Can. Res. 2004;6:119–127. doi: 10.1186/bcr781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuendet M, Pezzuto JM. Antitumor Activity of Bruceantin: An Old Drug with New Promise. Journal of Natural Products. 2004;67:269–272. doi: 10.1021/np030304+. [DOI] [PubMed] [Google Scholar]
- 11.Raynal NJ-M, Momparler L, Charbonneau M, Momparler RL. Antileukemic Activity of Genistein, a Major Isoflavone Present in Soy Products. Journal of Natural Products. 2008;71:3–7. doi: 10.1021/np070230s. [DOI] [PubMed] [Google Scholar]
- 12.Danilenko M, Wang X, Studzinski GP. Carnosic Acid and Promotion of Monocytic Differentiation of HL60-G Cells Initiated by Other Agents. J. Natl. Cancer Inst. 2001;93:1224–1233. doi: 10.1093/jnci/93.16.1224. [DOI] [PubMed] [Google Scholar]
- 13.Luyengi L, Suh N, Fong HHS, Pezzuto JM, Kinghorn AD. A lignan and four terpenoids from Brucea javanica that induce differentiation with cultured HL-60 promyelocytic leukemia cells. Phytochemistry. 1996;43:409–412. doi: 10.1016/0031-9422(96)00258-0. [DOI] [PubMed] [Google Scholar]
- 14.Cuendet M, Gills JJ, Pezzuto JM. Brusatol-induced HL-60 cell differentiation involves NF-kB activation. Cancer Lett. 2004;206:43–50. doi: 10.1016/j.canlet.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Mata-Greenwood E, Cuendet M, Sher D, Gustin D, Stock W, Pezzuto JM. Brusatol-mediated induction of leukemic cell differentiation and G1 arrest is associated with down-regulation of c-myc. Leukemia. 2002;16:2275–2284. doi: 10.1038/sj.leu.2402696. [DOI] [PubMed] [Google Scholar]
- 16.Tallman MS, Altman JK. Curative strategies in acute promyelocytic leukemia. Hematology. 2008;2008:391–399. doi: 10.1182/asheducation-2008.1.391. [DOI] [PubMed] [Google Scholar]
- 17.Talbot N, Rexroad C, Pursel V, Powell A. Alkaline phosphatase staining of pig and sheep epiblast cells in culture. Mol. Reprov. Dev. 1993;36:139–147. doi: 10.1002/mrd.1080360204. [DOI] [PubMed] [Google Scholar]
- 18.Pease S, Braghetta P, Gearing D, Grail D, Williams RL. Isolation of embryonic stem (ES) cells in media supplemented with recombinant leukemia inhibitory factor (LIF) Dev. Biol. 1990;141:344–352. doi: 10.1016/0012-1606(90)90390-5. [DOI] [PubMed] [Google Scholar]
- 19.Rivero-Cruz JF, Lezutekong R, Lobo-Echeverri T, Ito A, Mi Q, Chai H-B, Soejarto DD, Cordell GA, Pezzuto JM, Swanson SM, Morelli I, Kinghorn AD. Cytotoxic constituents of the twigs of Simarouba glauca collected from a plot in southern Florida. Phytother. Res. 2005;19:136–140. doi: 10.1002/ptr.1642. [DOI] [PubMed] [Google Scholar]
- 20.Raghunandan P, Reddy PN, Jayaveera KN. Characteristics and composition of Simarouba glauca D.C. seeds and fat. J. Oil Technol. Assoc. India. 2002;34:63–65. [Google Scholar]
- 21.Bhatnagar R, Patel JC, Shukla YM, Talati JG. Chemical composition of Simaruba glauca seed. Indian J. Agric. Biochem. 1998;11:58–59. [Google Scholar]
- 22.Bhatnagar S, Polonsky J, Prange T, Pascard C. New toxic quassinoid glucosides from Simarouba glauca. X-ray analysis. Tetrahedron Lett. 1984;25:299–302. [Google Scholar]
- 23.Ham EA, Schafer HM, Denkewalter RG, Brink NG. Structural studies on glaucarubin from Simarouba glauca. J. Am. Chem. Soc. 1954;76:6066–6068. [Google Scholar]
- 24.Ajaiyeoba EO, Krebs HC. Quafrinoic acids: Two new triterpenoids from Quassia africana stem bark. Niger. J. Nat. Prod. Med. 2003;7:39–41. [Google Scholar]
- 25.Apers S, Cimanga K, Vanden Berghe D, Van Meenen E, Longanga AO, Foriers A, Vlietinck A, Pieters L. Antiviral activity of simalikalactone D, a quassinoid from Quassia africana. Planta Med. 2002;68:20–24. doi: 10.1055/s-2002-19870. [DOI] [PubMed] [Google Scholar]
- 26.Ayafor JF, Tchuendem MK, Mbazoa CM, Ngadjui BT, Tillequin F. 13C NMR and other spectral data of 4-methylthiocanthin-6-one from Quassia africana. Bull. Chem. Soc. Ethiop. 1993;7:121–124. [Google Scholar]
- 27.Cabral JA, McChesney JD, Milhous WK. A new antimalarial quassinoid from Simaba guianensis. Journal of Natural Products. 1993;56:1954–1961. doi: 10.1021/np50101a014. [DOI] [PubMed] [Google Scholar]
- 28.Lumonadio L, Vanhaelen M. Indole alkaloids and quassin from Quassia africana. Journal of Natural Products. 1986;49:940. [Google Scholar]
- 29.Tresca JP, Alais L, Polonsky J. Bitter constituents of Quassia africana (Simarubaceae). Simalikalactones A, B, C, and D, and simalikahemiacetal A. C. R. Acad. Sci., Ser. C. 1971;273:601–604. [Google Scholar]
- 30.Franssen FFJ, Smeijsters L, Berger I, Aldana BEM. In vivo and in vitro antiplasmodial activities of some plants traditionally used in Guatemala against malaria. Antimicrob. Agents. 1997;41:1500–1503. doi: 10.1128/aac.41.7.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell RE, Geissman TA. Constituents of Suriana maritima. Triterpene diol of novel structure and new flavonol glycoside. Phytochemistry. 1971;10:1559–1567. [Google Scholar]
- 32.Hershenson BR, Quimby MW. Phytochemical investigation of the stem wood of Suriana maritima. J. Pharm. Sci. 1969;58:1411–1412. doi: 10.1002/jps.2600581126. [DOI] [PubMed] [Google Scholar]
- 33.Chuu C-P, Kokontis J, Hiipakka R, Liao S. Modulation of liver X receptor signaling as novel therapy for prostate cancer. J. Biomed. Sci. 2007;14:543–553. doi: 10.1007/s11373-007-9160-8. [DOI] [PubMed] [Google Scholar]
- 34.Chen AC, Gudas LJ. An Analysis of Retinoic Acid-induced Gene Expression and Metabolism in AB1 Embryonic Stem Cells. J. Biol. Chem. 1996;271:14971–14980. doi: 10.1074/jbc.271.25.14971. [DOI] [PubMed] [Google Scholar]
- 35.Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 1996;19:1518–1520. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- 36.Berridge MV, Herst PM, Tan AS, El-Gewely MR. Biotechnology Annual Review. Vol. 11. Elsevier; 2005. Tetrazolium dyes as tools in cell biology: New insights into their cellular reduction; pp. 127–152. [DOI] [PubMed] [Google Scholar]
- 37.Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, D'Armiento J, Weinstein IB, Kennelly EJ. Bioactive Depsides and Anthocyanins from Jaboticaba (Myrciaria cauliflora) Journal of Natural Products. 2006;69:1228–1230. doi: 10.1021/np0600999. [DOI] [PubMed] [Google Scholar]
- 38.Smith RC, Reeves JC, Dage RC, Schnettler RA. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem. Pharmacol. 1987;36:1457–1460. doi: 10.1016/0006-2952(87)90110-9. [DOI] [PubMed] [Google Scholar]
- 39.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 40.Gillespie RF, Gudas LJ. Retinoic acid receptor isotype specificity in F9 teratocarcinoma stem cells results from the differential recruitment of coregulators to retinoic response elements. J. Biol. Chem. 2007;282:33421–33434. doi: 10.1074/jbc.M704845200. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Ceballos E, Chambon P, Gudas LJ. Differences in gene expression between wild type and Hoxa1 knockout embryonic stem cells after retinoic acid treatment or leukemia inhibitory factor (LIF) removal. J. Biol. Chem. 2005;280:16484–16498. doi: 10.1074/jbc.M414397200. [DOI] [PubMed] [Google Scholar]
- 42.Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, Mongan NP. Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev. 2009;18:1093–1108. doi: 10.1089/scd.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. doi: 10.1038/nature06403. [DOI] [PubMed] [Google Scholar]
- 44.Eckfeldt CE, Mendenhall EM, Verfaillie CM. The molecular repertoire of the ‘almighty’ stem cell. Nat. Rev. Mol. Bio. 2005;6:726–737. doi: 10.1038/nrm1713. [DOI] [PubMed] [Google Scholar]
- 45.Rivero-Cruz JF, Lezutekong R, Lobo-Echeverri T, Ito A, Mi Q, Chai H, Soejarto D, Cordell GA, Pezzuto J, Swanson S, Morelli I, Kinghorn AD. Cytotoxic constituents of the twigs of Simarouba glauca collected from a plot in Southern Florida. Phytother. Res. 2005;19:136–140. doi: 10.1002/ptr.1642. [DOI] [PubMed] [Google Scholar]
- 46.Sharma M, Li L, Celver J, Killian C, Kovoor A, Seeram N. Effects of Fruit Ellagitannin Extracts, Ellagic Acid, and Their Colonic Metabolite, Urolithin A, on Wnt Signaling. J. Agric. Food Chem. 58:3965–3969. doi: 10.1021/jf902857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams LS, Phung S, Yee N, Seeram NP, Li L, Chen S. Blueberry Phytochemicals Inhibit Growth and Metastatic Potential of MDA-MB-231 Breast Cancer Cells through Modulation of the Phosphatidylinositol 3-Kinase Pathway. Cancer Res. 2010;70:3594–3605. doi: 10.1158/0008-5472.CAN-09-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nichenametla S, Taruscio T, Barney D, Exon J. A Review of the Effects and Mechanisms of Polyphenolics in Cancer. Crit. Rev. Food Sci. Nutr. 2006;46:161–183. doi: 10.1080/10408390591000541. [DOI] [PubMed] [Google Scholar]
- 49.Reynertson KA, Yang H, Jiang B, Basile MJ, Kennelly EJ. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chem. 2008;109:883–890. doi: 10.1016/j.foodchem.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He W, Van Puyvelde L, De Kimpe N, Verbruggen L, Anthonissen K, Van der Flaas M, Bosselaers J, Mathenge SG, Mudida FP. Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother. Res. 2002;16:66–70. doi: 10.1002/ptr.849. [DOI] [PubMed] [Google Scholar]
- 51.Thouvenel C, Gantier JC, Duret P, Fourneau C, Hocquemiller R, Ferreira ME, Rojas de Arias A, Fournet A. Antifungal compounds from Zanthoxylum chiloperone var. angustifolium. Phytother. Res. 2003;17:678–680. doi: 10.1002/ptr.1137. [DOI] [PubMed] [Google Scholar]
- 52.Chen JJ, Fang HY, Duh CY, Chen IS. New indolopyridoquinazoline, benzo[c]phenanthridines and cytotoxic constituents from Zanthoxylum integrifoliolum. Planta Med. 2005;71:470–475. doi: 10.1055/s-2005-864144. [DOI] [PubMed] [Google Scholar]
- 53.Kuo PC, Shi LS, Damu AG, Su CR, Huang CH, Ke CH, Wu JB, Lin AJ, Bastow KF, Lee KH, Wu TS. Cytotoxic and antimalarial beta-carboline alkaloids from the roots of Eurycoma longifolia. Journal of Natural Products. 2003;66:1324–1327. doi: 10.1021/np030277n. [DOI] [PubMed] [Google Scholar]
- 54.Kwak JH, Kang MW, Roh JH, Choi SU, Zee OP. Cytotoxic phenolic compounds from Chionanthus retusus. Arch. Pharm. Res. 2009;32:1681–1687. doi: 10.1007/s12272-009-2203-0. [DOI] [PubMed] [Google Scholar]
- 55.DellaGreca M, Cutillo F, D'Abrosca B, Fiorentino A, Pacifico S, Zarrelli A. Antioxidant and radical scavenging properties of Malva sylvestris. Nat. Prod. Comm. 2009;4:893–896. [PubMed] [Google Scholar]
- 56.Pan R, Gao XH, Li Y, Xia YF, Dai Y. Anti-arthritic effect of scopoletin, a coumarin compound occurring in Erycibe obtusifolia Benth stems, is associated with decreased angiogenesis in synovium. Fundam. Clin. Pharmacol. 2009 doi: 10.1111/j.1472-8206.2009.00784.x. in press, DOI: 10.1111/j.1472-8206.2009.00784.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.