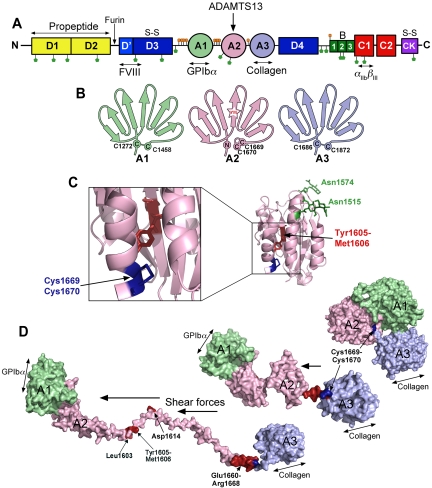
Figure 1.
The substrate, VWF. (A) Domain organization of VWF. N-linked and O-linked glycosylation sites are represented by green and orange lollipops, respectively. The propeptide (yellow represents D1 and D2) is marked. S-S indicates sites of intermolecular disulphide bond pairing. Vertical arrows indicate the location of cleavage sites (furin and ADAMTS13). Ligand binding sites (FVIII, GPIbα, collagen, and αIIbβIII) are labeled below. (B) Schematic representation of the VWF A1, A2, and A3 domains. The paired cysteines in A1 and A3 are shown. The vicinal disulphide bond that forms the molecular plug in the A2 domain and the Tyr1605-Met1606 (YM) ADAMTS13 cleavage site are also represented. (C) Structure of the VWF A2 domain highlighting the N-linked glycosylation sites (green), vicinal disulphide bond (blue), and the ADAMTS13 cleavage site (red) hidden in the center of the folded domain. (D) Molecular models of the unfolding of the VWF A1-A2-A3 domains. In globular VWF, the A3 domain collagen binding site is exposed. Elevated shear forces on VWF cause uncoupling of the A domains, extraction of the Cys1669-Cys1670 vicinal disulphide plug, and unraveling of the A2 domain. This exposes the GPIbα binding site in the A1 domain, cryptic ADAMTS13 binding sites, and the cleavage site in the A2 domain (red).