Figure 5.
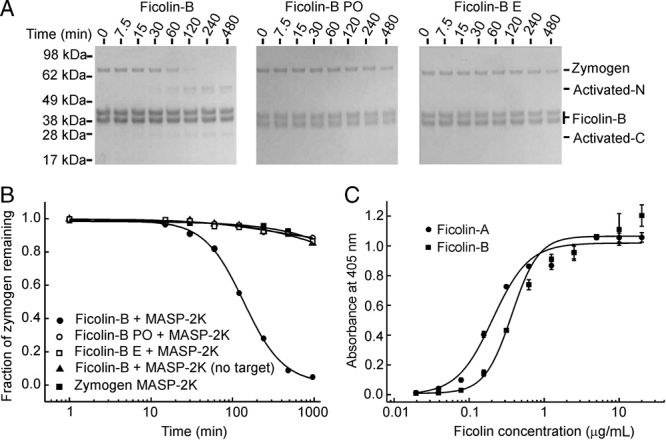
Kinetics of MASP-2 activation by ficolin-B and ficolin-B mutants. Activation of MASP-2K by WT and mutant ficolins on GlcNAc-Sepharose as an activating target. (A) Aliquots of protein complexes taken at different time points were analyzed by SDS-PAGE (10% gel), stained with Coomassie blue. The migration positions of zymogen MASP-K and the N- and C-terminal fragments of activated MASP-2K (activated –N and –C) are indicated. (B) The amount of activation was determined by measuring cleavage of zymogen MASP-2K. Only 12% cleavage was observed for either mutant after 16 h comparable to the amount of autoactivation of MASP-2K alone. In ficolin-B PO, Lys54 and Ala55 have been mutated to proline and hydroxyproline, respectively. In ficolin-B E, Ala55 was replaced by a glutamic acid residue. (C) Complement activation by recombinant rat ficolins-A and -B. Complement activation was measured on acetylated-BSA using an alkaline phosphatase-conjugated antibody specific for the membrane attack complex, following incubation of chromogenic substrate. Background absorbance, in the absence of ficolin, was subtracted from the data. Data are the average±SE from duplicate observations.
